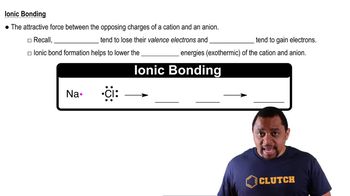
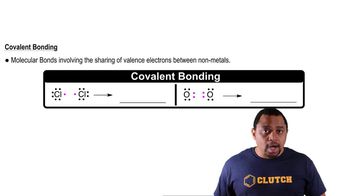
Textbook Question
Electrostatic potential maps of acetaldehyde (C2H4), ethane (C2H6), ethanol (C2H6O), and fluorethane (C2H5F) are shown. Which do you think is which? (a)
(b)
(c)
(d)

 Verified step by step guidance
Verified step by step guidance

Electrostatic potential maps of acetaldehyde (C2H4), ethane (C2H6), ethanol (C2H6O), and fluorethane (C2H5F) are shown. Which do you think is which? (a)
(b)
(c)
(d)
What general trends in electronegativity occur in the periodic table?