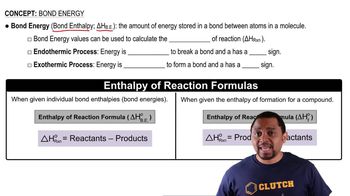
Textbook Question
The following ball-and-stick molecular model is a representation of thalidomide, a drug that causes birth defects when taken by expectant mothers but is valuable for its use against leprosy. The lines indicate only the connections between atoms, not whether the bonds are single, double, or triple. (Red = O, gray = C, blue = N, ivory = H). Give the formula of thalidomide, and indicate the positions of multiple bonds and lone pairs.