Textbook Question
Which of the following alkali metal halides has the largest lat-tice energy? Explain.(a) (b) (c)


 Verified step by step guidance
Verified step by step guidance
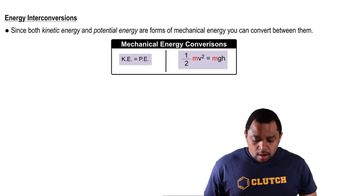


Electrostatic potential maps of acetaldehyde (C2H4), ethane (C2H6), ethanol (C2H6O), and fluorethane (C2H5F) are shown. Which do you think is which? (a)
(b)
(c)
(d)