 Back
BackProblem 7c2
Consider the three electronic transitions in a hydrogen atom shown here, labeled A, B, and C. (c) Calculate the wavelength of the photon emitted for each transition. Do any of these transitions lead to the emission of visible light? If so which one(s)?

Calculate the wavelength of the photon emitted for transition B.
Problem 8b
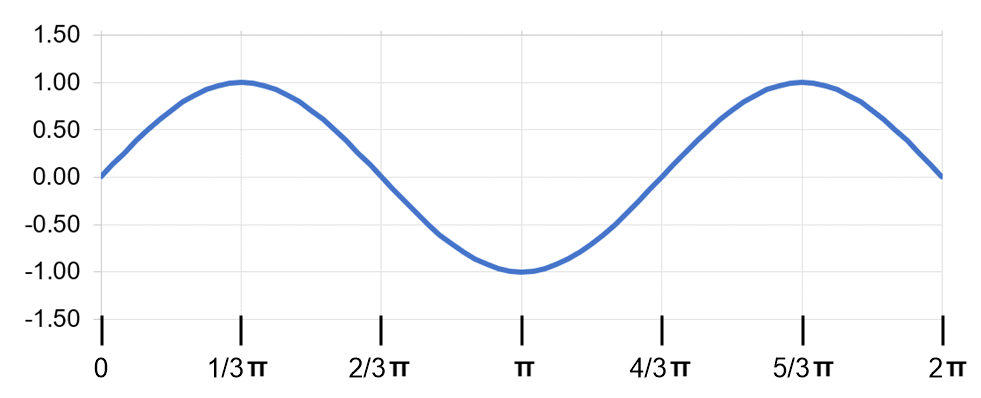
Consider a fictitious one-dimensional system with one electron. The wave function for the electron, drawn below, is c1x2 = sin x from x = 0 to x = 2p. (b) At what value or values of x will there be the greatest probability of finding the electron?
Problem 9a
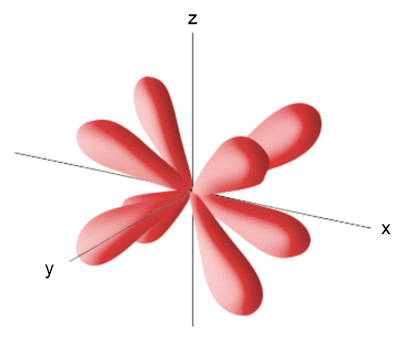
The contour representation of one of the orbitals for the n = 3 shell of a hydrogen atom is shown here. (a) What is the quantum number l for this orbital?

Problem 9c
The contour representation of one of the orbitals for the n = 3 shell of a hydrogen atom is shown here. (c) In which of the following ways would you modify this sketch if the value of the magnetic quantum number, ml, were to change? (i) It would be drawn larger, (ii) the number of lobes would change, (iii) the lobes of the orbital would point in a different direction, (iv) there would be no change in the sketch.
Problem 10b
The accompanying drawing shows a contour plot for a dyz orbital. Consider the quantum numbers that could potentially correspond to this orbital. (b) What is the value of the angular momentum quantum number, l?
Problem 10c
The accompanying drawing shows a contour plot for a dyz orbital. Consider the quantum numbers that could potentially correspond to this orbital. (c) What is the largest possible value of the magnetic quantum number, ml?
Problem 10d
The accompanying drawing shows a contour plot for a dyz orbital. Consider the quantum numbers that could potentially correspond to this orbital. (d) The probability density goes to zero along which of the following planes: xy, xz, or yz?

Problem 11a
Four possible electron configurations for a carbon atom are shown below, but only one schematic represents the correct configuration for a carbon atom in its ground state. Which one is the correct electron configuration?

Problem 11b
Four possible electron configurations for a nitrogen atom are shown below, but only one schematic represents the correct configuration for a nitrogen atom in its ground state. Which configurations violate the Pauli exclusion principle?
Problem 12
State where in the periodic table these elements appear: (a) elements with the valence-shell electron configuration ns2np5 (b) elements that have three unpaired p electrons (c) an element whose valence electrons are 4s24p1 (d) the d-block elements [Section 6.9]
- The wavenumber l is the number of waves that exist over a specified distance, very often 1 cm. The wavenumber can easily be calculated by taking the reciprocal of the wavelength. Give typical wavenumbers for (a) X-rays (λ = 1 nm) (b) visible light (λ = 500 nm) (c) microwaves (λ = 1 mm).
Problem 13
- Carbon dioxide in the atmosphere absorbs energy in the 4.0–4.5 mm range of the spectrum. (a) Calculate the frequency of the 4.0 mm radiation.
Problem 14
Problem 15a
Label each of the following statements as true or false. a. Visible light is a form of electromagnetic radiation.
Problem 15b
Label each of the following statements as true or false. b. Ultraviolet light has longer wavelengths than visible light.
Problem 15c
Label each of the following statements as true or false. c. X rays travel faster than microwaves.
Problem 16
Label each of the following statements as true or false. a. The frequency of radiation increases as the wavelength increases. b. Electromagnetic radiation travels through a vacuum at a constant speed, regardless of wavelength. c. Infrared light has higher frequencies than visible light. d. The glow from a fireplace, the energy within a microwave oven, and a foghorn blast are all forms of electromagnetic radiation.
Problem 17
Arrange the following kinds of electromagnetic radiation in order of increasing wavelength: infrared light, green light, red light, radio waves, X rays, ultraviolet light.
- List the following types of electromagnetic radiation in order of increasing wavelength: (a) the gamma rays produced by a radioactive nuclide used in medical imaging; (b) radiation from an FM radio station at 93.1 MHz on the dial; (c) a radio signal from an AM radio station at 680 kHz on the dial; (d) the yellow light from sodium vapor streetlights; (e) the red light of a light-emitting diode, such as in a calculator display.
Problem 18
Problem 19a
(a) What is the frequency of radiation that has a wavelength of 10 µm, about the size of a bacterium?
Problem 20d
(d) What distance does electromagnetic radiation travel in 0.38 ps?
Problem 21a
A laser pointer used in a lecture hall emits light at 650 nm. What is the frequency of this radiation?
Problem 21b
A laser pointer used in a lecture hall emits light at 650 nm. Using Figure 6.4, predict the color associated with this wavelength.
Problem 22
It is possible to convert radiant energy into electrical energy using photovoltaic cells. Assuming equal efficiency of conversion, would infrared or ultraviolet radiation yield more electrical energy on a per-photon basis?
Problem 23
If human height were quantized in 1-foot increments, what would happen to the height of a child as she grew up?
a. The child’s height would never change.
b. The child’s height would continuously get greater.
c. The child’s height would increase in “jumps” of 1 foot at a time.
d. The child’s height would increase in jumps of 6 inches.
Problem 25a
(a) Calculate the energy of a photon of electromagnetic radiation whose frequency is 2.94 × 1014 s-1.
Problem 25b
(b) Calculate the energy of a photon of radiation whose wavelength is 413 nm.
Problem 26a
(a) A green laser pointer emits light with a wavelength of 532 nm. What is the frequency of this light?
Problem 26b
(b) What is the energy of one of these photons?
Problem 26c
(c) The laser pointer emits light because electrons in the material are excited (by a battery) from their ground state to an upper excited state. When the electrons return to the ground state, they lose the excess energy in the form of 532-nm photons. What is the energy gap between the ground state and excited state in the laser material?
- (a) Calculate and compare the energy of a photon with a wavelength of 3.0 mm to that of a photon with a wavelength of 0.3 nm.
Problem 27
