Textbook Question
(b) Calculate the energy of a photon of radiation whose wavelength is 413 nm.
1
views

 Verified step by step guidance
Verified step by step guidance

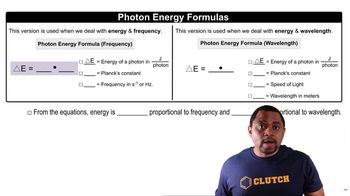

(b) Calculate the energy of a photon of radiation whose wavelength is 413 nm.
(a) A green laser pointer emits light with a wavelength of 532 nm. What is the frequency of this light?
(b) What is the energy of one of these photons?
An AM radio station broadcasts at 1010 kHz, and its FM partner broadcasts at 98.3 MHz. Calculate and compare the energy of the photons emitted by these two radio stations.
One type of sunburn occurs on exposure to UV light of wavelength in the vicinity of 325 nm. (c) How many photons are in a 1.00 mJ burst of this radiation?