The accompanying drawing shows a contour plot for a dyz orbital. Consider the quantum numbers that could potentially correspond to this orbital. (c) What is the largest possible value of the magnetic quantum number, ml?
Ch.6 - Electronic Structure of Atoms

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 6, Problem 11b
Four possible electron configurations for a nitrogen atom are shown below, but only one schematic represents the correct configuration for a nitrogen atom in its ground state. Which configurations violate the Pauli exclusion principle?
 Verified step by step guidance
Verified step by step guidance1
Identify the electron configurations provided for the nitrogen atom. Nitrogen has 7 electrons in total.
Recall the Pauli Exclusion Principle, which states that no two electrons in an atom can have the same set of four quantum numbers. This means each orbital can hold a maximum of two electrons with opposite spins.
Examine each electron configuration to check if any orbital is shown to contain more than two electrons or if electrons in the same orbital have the same spin direction.
Identify configurations where orbitals violate the Pauli Exclusion Principle by either having more than two electrons or electrons with the same spin in one orbital.
Eliminate the configurations that do not adhere to the Pauli Exclusion Principle to find the correct ground state configuration for a nitrogen atom.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Electron Configuration
Electron configuration describes the distribution of electrons in an atom's orbitals. For nitrogen, which has seven electrons, the correct ground state configuration is 1s² 2s² 2p³. Understanding how electrons fill orbitals according to energy levels and sublevels is crucial for identifying valid configurations.
Recommended video:
Guided course

Electron Configuration Example
Pauli Exclusion Principle
The Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers. This means that an orbital can hold a maximum of two electrons, which must have opposite spins. Recognizing violations of this principle helps in determining incorrect electron configurations.
Recommended video:
Guided course
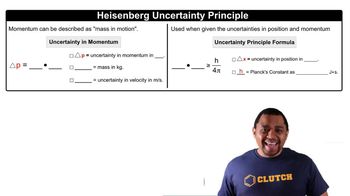
Uncertainty Principle Formula
Hund's Rule
Hund's rule states that electrons will occupy degenerate orbitals singly before pairing up. This minimizes electron-electron repulsion and leads to a more stable arrangement. Understanding this rule is essential for correctly filling the p orbitals in nitrogen's electron configuration.
Recommended video:
Guided course

Solubility Rules
Related Practice
Textbook Question
Textbook Question
The accompanying drawing shows a contour plot for a dyz orbital. Consider the quantum numbers that could potentially correspond to this orbital. (d) The probability density goes to zero along which of the following planes: xy, xz, or yz?
Textbook Question
Four possible electron configurations for a carbon atom are shown below, but only one schematic represents the correct configuration for a carbon atom in its ground state. Which one is the correct electron configuration?
Textbook Question
State where in the periodic table these elements appear: (a) elements with the valence-shell electron configuration ns2np5 (b) elements that have three unpaired p electrons (c) an element whose valence electrons are 4s24p1 (d) the d-block elements [Section 6.9]
