It takes 4 h 39 min for a 2.00-mg sample of radium-230 to decay to 0.25 mg. What is the half-life of radium-230?
Ch.21 - Nuclear Chemistry

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 21, Problem 39
Radium-226, which undergoes alpha decay, has a half-life of 1600 yr. (a) How many alpha particles are emitted in 5.0 min by a 10.0-mg sample of 226Ra?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Determine the number of moles of radium-226 in the 10.0 mg sample. Use the molar mass of radium-226, which is approximately 226 g/mol, to convert the mass to moles.
Step 2: Calculate the initial number of radium-226 atoms in the sample using Avogadro's number (6.022 x 10^23 atoms/mol).
Step 3: Use the half-life formula to find the decay constant (\( \lambda \)) for radium-226. The formula is \( \lambda = \frac{0.693}{\text{half-life}} \). Convert the half-life from years to minutes for consistency with the time period given.
Step 4: Apply the first-order decay equation \( N = N_0 e^{-\lambda t} \) to find the number of radium-226 atoms remaining after 5.0 minutes. Here, \( N_0 \) is the initial number of atoms, \( \lambda \) is the decay constant, and \( t \) is the time in minutes.
Step 5: Subtract the number of remaining radium-226 atoms from the initial number to find the number of atoms that decayed. Since each decay corresponds to the emission of one alpha particle, this number is also the number of alpha particles emitted.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Alpha Decay
Alpha decay is a type of radioactive decay in which an unstable nucleus emits an alpha particle, consisting of two protons and two neutrons. This process reduces the atomic number by two and the mass number by four, transforming the original element into a different element. Understanding alpha decay is crucial for calculating the number of emitted particles over a given time period.
Recommended video:
Guided course
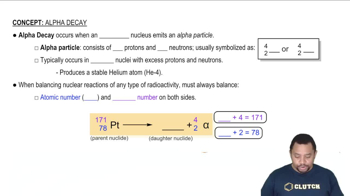
Alpha Decay
Half-Life
The half-life of a radioactive substance is the time required for half of the radioactive nuclei in a sample to decay. For Radium-226, the half-life is 1600 years, meaning that after this period, half of the original amount will have decayed. This concept is essential for determining how much of the sample remains and how many decay events occur over a specified time frame.
Recommended video:
Guided course

Zero-Order Half-life
Radioactive Decay Rate
The decay rate of a radioactive substance is the frequency at which its nuclei decay, typically expressed in terms of the number of decays per unit time. This rate can be calculated using the initial quantity of the substance, its half-life, and the time elapsed. In this question, it is necessary to convert the time from minutes to years to accurately apply the half-life in determining the number of alpha particles emitted.
Recommended video:
Guided course

Rate of Radioactive Decay
Related Practice
Textbook Question
Textbook Question
Cobalt-60 is a strong gamma emitter that has a half-life of 5.26 yr. The cobalt-60 in a radiotherapy unit must be replaced when its radioactivity falls to 75% of the original sample. If an original sample was purchased in June 2021, when will it be necessary to replace the cobalt-60?
Textbook Question
The cloth shroud from around a mummy is found to have a 14C activity of 9.7 disintegrations per minute per gram of carbon as compared with living organisms that undergo 16.3 disintegrations per minute per gram of carbon. From the half-life for 14C decay, 5730 yr, calculate the age of the shroud.
Textbook Question
Potassium-40 decays to argon-40 with a half-life of 1.27 * 109 yr. What is the age of a rock in which the mass ratio of 40Ar to 40K is 4.2?
