Energy bands are considered continuous due to the large number of closely spaced energy levels. The range of energy levels in a crystal of copper is approximately 1×10−19 J. Assuming equal spacing between levels, one can approximate the spacing between energy levels by dividing the range of energies by the number of atoms in the crystal. b. Determine the average spacing in J between energy levels in the copper metal in part (a).

Hydrogen bonding between polyamide chains plays an important role in determining the properties of a nylon such as nylon 6,6 (Table 12.6). Draw the structural formulas for two adjacent chains of nylon 6,6 and show where hydrogen-bonding interactions could occur between them.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
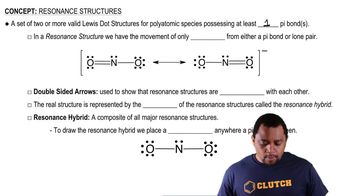
Hydrogen Bonding
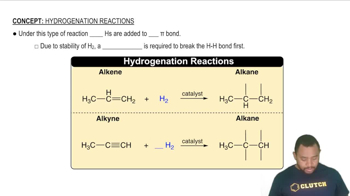
Polyamide Structure
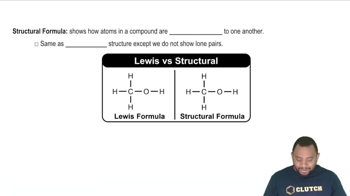
Structural Formulas
Sodium oxide (Na2O) adopts a cubic structure with Na atoms represented by green spheres and O atoms by red spheres.
(c) The unit cell edge length is 5.550 Å. Determine the density of Na2O.
In their study of X-ray diffraction, William and Lawrence Bragg determined that the relationship among the wavelength of the radiation 1l2, the angle at which the radiation is diffracted 1u2, and the distance between planes of atoms in the crystal that cause the diffraction (d) is given by nl = 2d sin u. X rays from a copper X-ray tube that have a wavelength of 1.54 Å are diffracted at an angle of 14.22 degrees by crystalline silicon. Using the Bragg equation, calculate the distance between the planes of atoms responsible for diffraction in this crystal, assuming n = 1 (first-order diffraction).
Germanium has the same structure as silicon, but the unit cell size is different because Ge and Si atoms are not the same size. If you were to repeat the experiment described in Additional Exercise 12.117, but replace the Si crystal with a Ge crystal, would you expect the X rays to be diffracted at a larger or smaller angle 𝜃?
(a) The density of diamond is 3.5 g/cm3, and that of graphite is 2.3 g/cm3. Based on the structure of buckminsterfullerene, what would you expect its density to be relative to these other forms of carbon?
