Back
BackProblem 64
A heat engine with 0.20 mol of a monatomic ideal gas initially fills a 2000 cm³ cylinder at 600 K. The gas goes through the following closed cycle: Isothermal expansion to 4000 cm³. Isochoric cooling to 300 K. Isothermal compression to 2000 cm³. Isochoric heating to 600 K. How much work does this engine do per cycle and what is its thermal efficiency?
Problem 69
100 mL of water at 15℃ is placed in the freezer compartment of a refrigerator with a coefficient of performance of 4.0. How much heat energy is exhausted into the room as the water is changed to ice at -15℃?
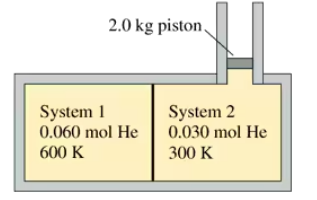
Problem 70b
FIGURE CP21.70 shows two insulated compartments separated by a thin wall. The left side contains 0.060 mol of helium at an initial temperature of 600 K and the right side contains 0.030 mol of helium at an initial temperature of 300 K. The compartment on the right is attached to a vertical cylinder, above which the air pressure is 1.0 atm. A 10-cm-diameter, 2.0 kg piston can slide without friction up and down the cylinder. Neither the cylinder diameter nor the volumes of the compartments are known. How much heat is transferred from the left side to the right side?
Problem 73a
The gasoline engine in your car can be modeled as the Otto cycle shown in FIGURE CP21.73. A fuel-air mixture is sprayed into the cylinder at point 1, where the piston is at its farthest distance from the spark plug. This mixture is compressed as the piston moves toward the spark plug during the adiabatic compression stroke. The spark plug fires at point 2, releasing heat energy that had been stored in the gasoline. The fuel burns so quickly that the piston doesn't have time to move, so the heating is an isochoric process. The hot, high-pressure gas then pushes the piston outward during the power stroke. Finally, an exhaust value opens to allow the gas temperature and pressure to drop back to their initial values before starting the cycle over again. Analyze the Otto cycle and show that the work done per cycle is