Ch. 15 - Structural Identification II: Nuclear Magnetic Resonance Spectroscopy
 Back
BackAll textbooks Mullins 1st Edition
Mullins 1st Edition Ch. 15 - Structural Identification II: Nuclear Magnetic Resonance Spectroscopy
Ch. 15 - Structural Identification II: Nuclear Magnetic Resonance Spectroscopy
 Mullins 1st Edition
Mullins 1st Edition Ch. 15 - Structural Identification II: Nuclear Magnetic Resonance Spectroscopy
Ch. 15 - Structural Identification II: Nuclear Magnetic Resonance SpectroscopyProblem 2
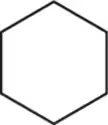
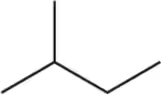
Radical chlorination, a reaction studied in Chapters 5 and 11, replaces a hydrogen with a chlorine. Assuming there is no regioselectivity (all hydrogens can react equally), how many different chloroalkanes are possible upon reaction of each alkane with one equivalent of Cl₂
(a)
(b)
(c)
(d)
Problem 3
(a) Rank the following bonds in terms of the strength of their bond dipole (1 = weakest, 6 = strongest).
(b) Which carbon has the largest δ⁺ ?
C―F, C―Br, C―I, C―H, C―C, C―Cl
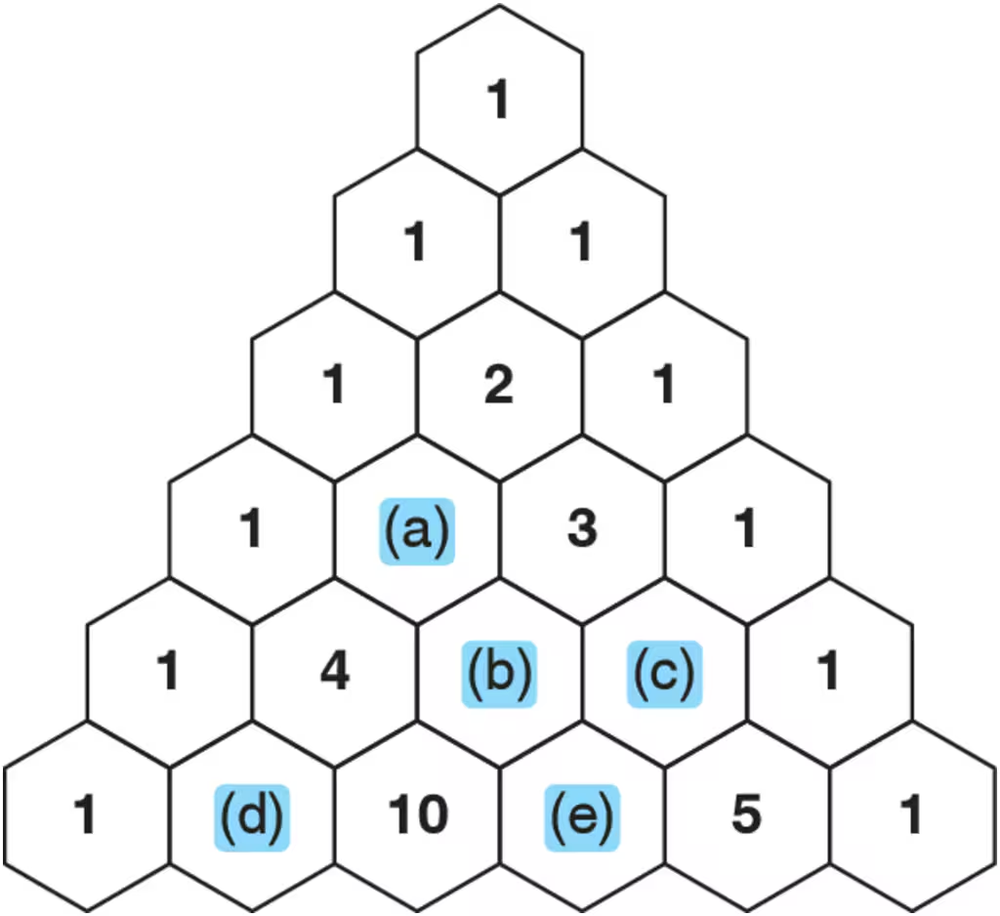
Problem 4
Every number in Pascal’s triangle is the sum of the two numbers above it. Given this, fill in the missing numbers.