Back
Back Mullins 1st Edition
Mullins 1st Edition Ch. 14 - Structural Identification I: Infrared Spectroscopy and Mass Spectrometry
Ch. 14 - Structural Identification I: Infrared Spectroscopy and Mass SpectrometryProblem 1a
Given the molecular formula, calculate the index of hydrogen deficiency.
(a) C6H14O
Problem 1b
Given the molecular formula, calculate the index of hydrogen deficiency.
(b) C4H9ON
Problem 1d
Given the molecular formula, calculate the index of hydrogen deficiency.
(d) C8H9OBr
Problem 2a(i)
Given the molecular formula, (i) what functional groups are possible in each of the following molecules?
(a) C6H14O
Problem 2b(i)
Given the molecular formula, (i) what functional groups are possible in each of the following molecules?
(b) C6H10O
Problem 3
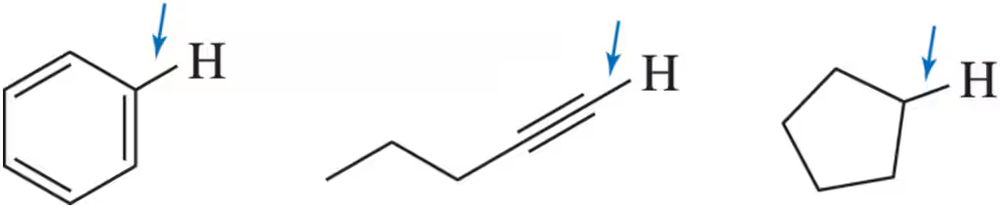
Rank C―H σ the following bonds in terms of their bond length. Explain your ranking ( 1= longest ; 3 = shortest).
Problem 4
In the periodic table, the number 79.904 appears under the element bromine. What is the significance of this number?
Problem 5
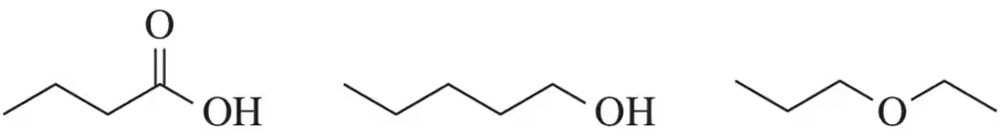
Rank the boiling points of the following molecules. Explain your ranking ( 1 = highest bp; 3 = lowest bp ).
Problem 6a
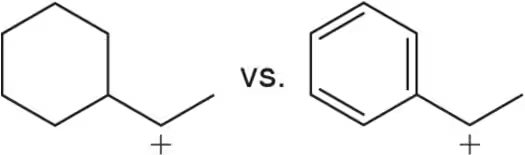
Identify the more stable carbocation in each pair.
(a)
Problem 6b
Identify the more stable carbocation in each pair.
(b)
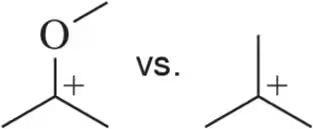
Problem 6c
Identify the more stable carbocation in each pair.
(c)
Problem 11
What functional groups are present in a molecule with a molecular formula of C₄H₁₀O and the following IR spectrum? [Use Table 14.2 for this.]
<IMAGE>
Problem 13b
Given the IR spectrum, suggest what functional groups might correspond to the given molecular formula.
(b) C3H7NO
<IMAGE>
Problem 13d
Given the IR spectrum, suggest what functional groups might correspond to the given molecular formula.
(d) C₇H₅O
<IMAGE>
Problem 15a
Choose the bond in each pair that you expect to vibrate at the higher wavenumber.
(a) C―N vs. C = N
Problem 15b
Choose the bond in each pair that you expect to vibrate at the higher wavenumber.
(b) C―H vs. C―O
Problem 16a
Calculate the reduced mass for the following bonds.
(a) C―H
Problem 16c
Calculate the reduced mass for the following bonds.
(c) C―Cl
Problem 18a
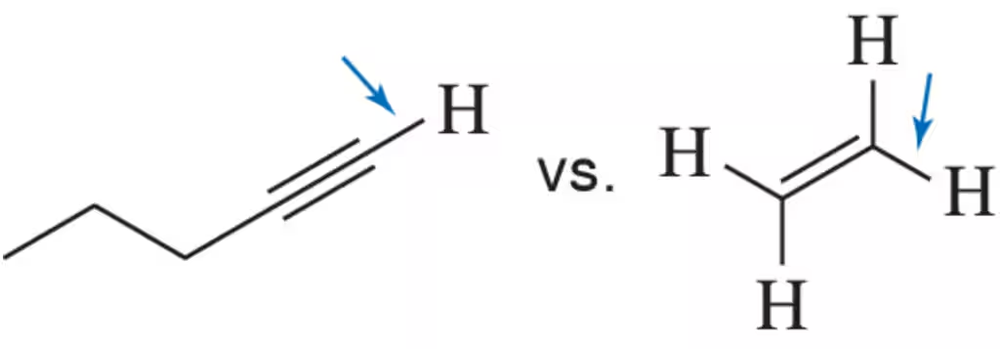
Would you expect an acetylenic C―H to absorb at a higher or lower wavenumber than the C―H in ethene?
Problem 19a
The IR spectrum for anisole contains two C―O stretching bands in the fingerprint region. Match the band to the bond that gives rise to it. Why are these bands so intense?
<IMAGE>
Problem 20d
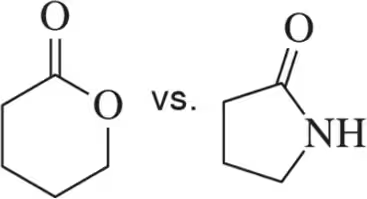
For each pair, choose the molecule that you expect to have the highest wavenumber for its C=O stretch.
(d)
Problem 22c
Match each of the four IR spectra to one of the given compounds. [One of the compounds does not match a spectrum.]
(c) <IMAGE>
Problem 22d
Match each of the four IR spectra to one of the given compounds. [One of the compounds does not match a spectrum.]
(d) <IMAGE>
Problem 29
The molecule that gave the mass spectrum shown here contains a halogen. Which halogen is present?
<IMAGE>