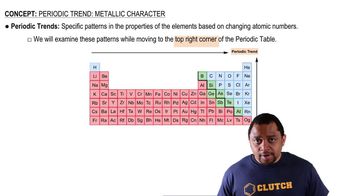
Effective Nuclear Charge
Effective nuclear charge (Z_eff) is the net positive charge experienced by an electron in a multi-electron atom. It accounts for the shielding effect of inner electrons, which reduces the full nuclear charge felt by outer electrons. A higher Z_eff leads to greater attraction between the nucleus and outer electrons, resulting in higher ionization energy, as seen in the comparison of Ca, K, and Mg.

 Verified step by step guidance
Verified step by step guidance