Write the balanced chemical equation for the dissociation of each of the following carboxylic acids in water:
a. pentanoic acid

 Verified step by step guidance
Verified step by step guidance
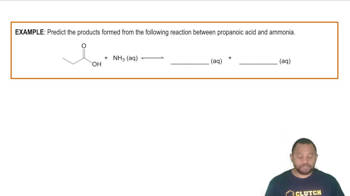
Write the balanced chemical equation for the dissociation of each of the following carboxylic acids in water:
a. pentanoic acid
Write the balanced chemical equation for the dissociation of each of the following carboxylic acids in water:
b. butanoic acid
Write the balanced chemical equation for the reaction of each of the following carboxylic acids with NaOH:
c. benzoic acid
Write the IUPAC and common names, if any, of the carboxylate salts produced in problem 14.11
a. formic acid
b. 3-chloropropanoic acid
c. benzoic acid
Write the IUPAC and common names, if any, of the carboxylate salts produced in problem 14.12
a. acetic acid
b. 2-methylbutanoic acid
c. 4-chlorobenzoic acid
Draw the condensed structural formula for the ester formed when each of the following reacts with methyl alcohol:
b. pentanoic acid