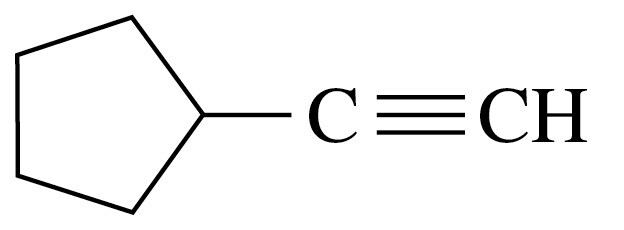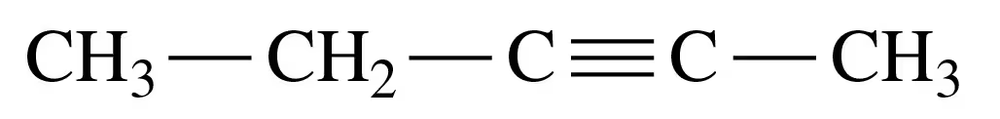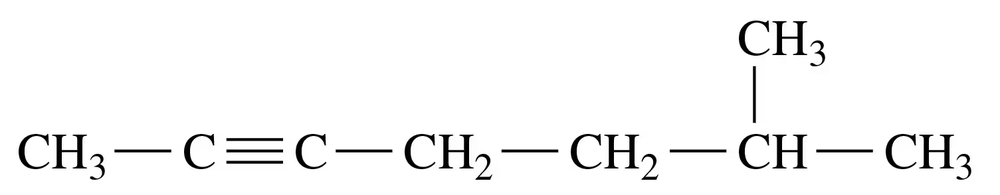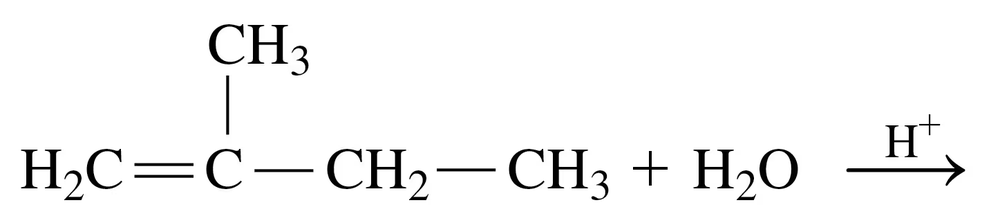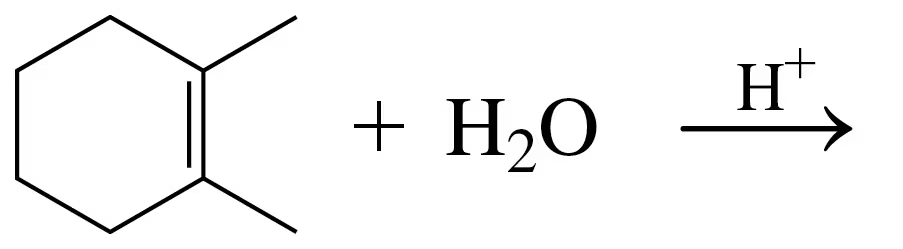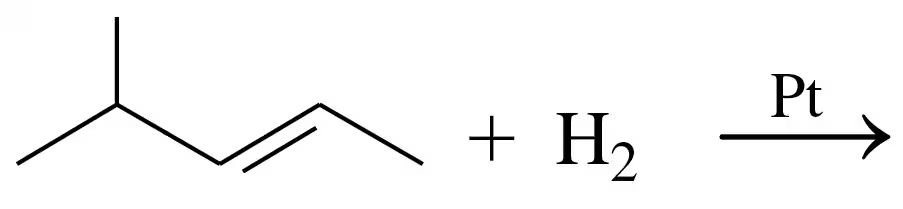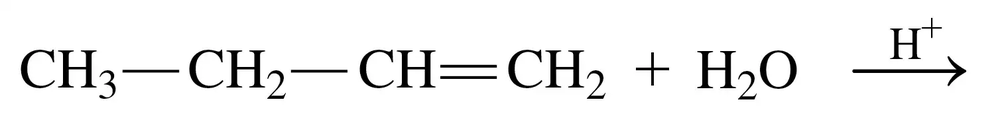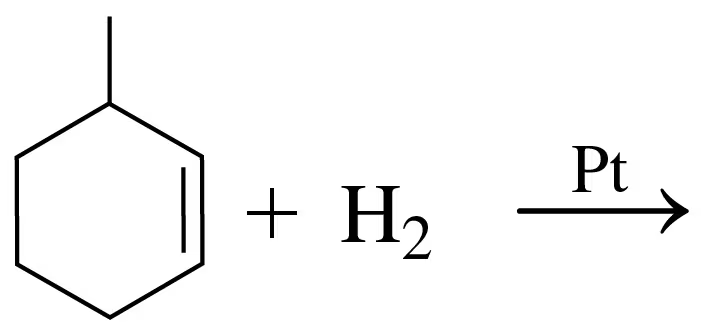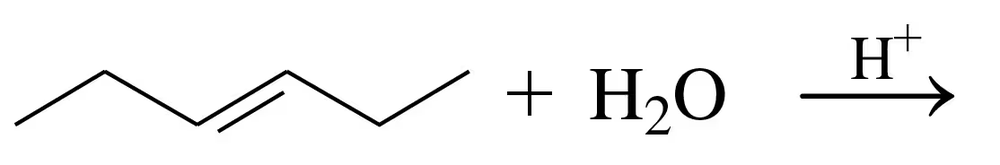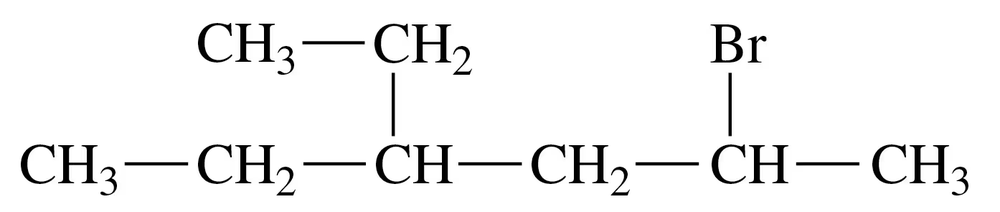Back
BackProblem 24d
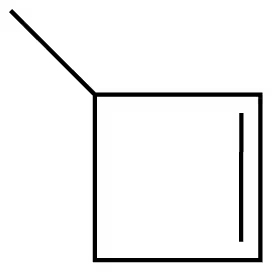
Identify the following as alkanes, alkenes, cycloalkenes, or alkynes:
d.
Problem 25c
Give the IUPAC name for each of the following:
c.
Problem 25d
Give the IUPAC name for each of the following:
d.
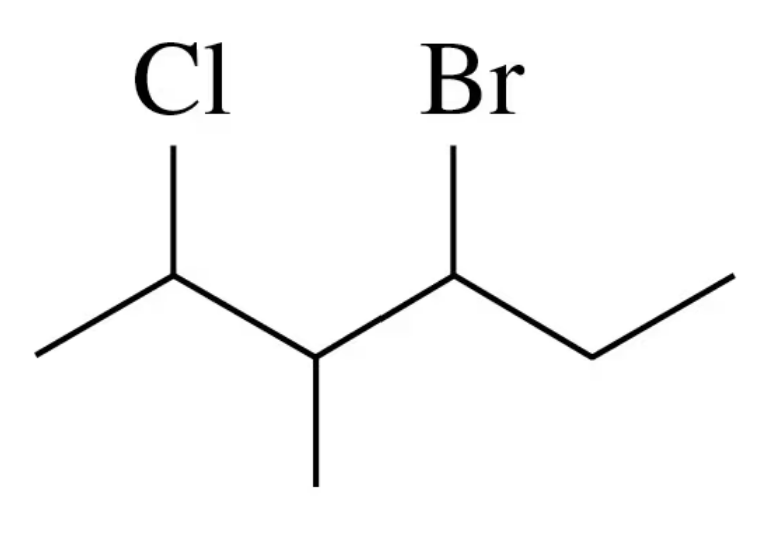
Problem 26b
Give the IUPAC name for each of the following:
b.
Problem 27d
Draw the condensed structural formula, or line-angle formula, if cyclic, for each of the following:
d. 3-chloro-1-butyne
Problem 28a
Draw the condensed structural formula, or line-angle formula, if cyclic, for each of the following:
a. 1-methylcyclopentene
Problem 28c
Draw the condensed structural formula, or line-angle formula, if cyclic, for each of the following:
b. 1-bromo-3-hexyne
Problem 31c
Draw the condensed structural formula for each of the following:
a. trans-1-bromo-2-chloroethene
Problem 32a
Draw the condensed structural formula for each of the following:
a. cis-1,2-difluoroethene
Problem 32b
Draw the condensed structural formula for each of the following:
b. trans-2-pentene
Problem 33b
Draw the structural formula for the product in each of the following reactions:
b.
Problem 33d
Draw the structural formula for the product in each of the following reactions:
d.
Problem 34a
Draw the structural formula for the product in each of the following reactions:
b.
Problem 34b
Draw the structural formula for the product in each of the following reactions:
a.
Problem 34c
Draw the structural formula for the product in each of the following reactions:
c.
Problem 34d
Draw the structural formula for the product in each of the following reactions:
d.
Problem 35b
Give the IUPAC name for each of the following:
b.
Problem 36a
Give the IUPAC name for each of the following:
c.
Problem 36b
Give the IUPAC name for each of the following:
b.
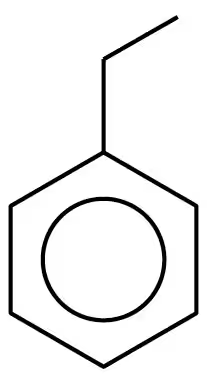
Problem 37c
Draw the line-angle formula for each of the following compounds:
c. 4-ethyltoluene
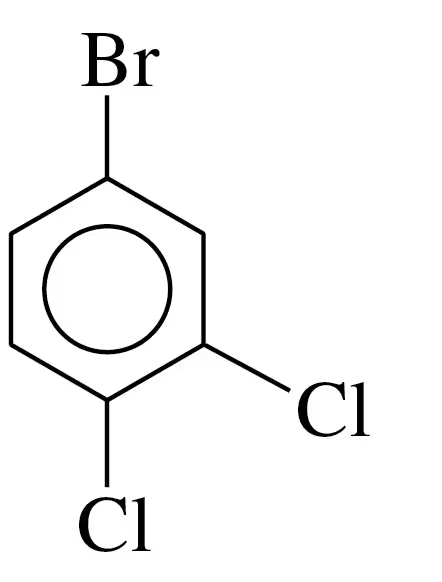
Problem 38c
Draw the line-angle formula for each of the following compounds:
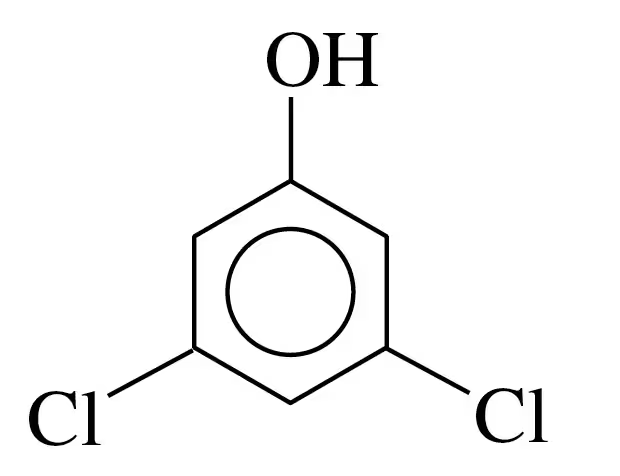
c. 1,2,4-trichlorobenzene
Problem 39c
Write the balanced chemical equation for the complete combustion of each of the following hydrocarbons found in gasoline:
c. 3-ethyltoluene
Problem 41a
Match the following physical and chemical properties with potassium chloride, KCl, used in salt substitutes, or butane, C4H10 used in lighters:
<IMAGE>
a. melts at -138°C
Problem 41e
Match the following physical and chemical properties with potassium chloride, KCl, used in salt substitutes, or butane, C4H10 used in lighters:
<IMAGE>
e. is a gas at room temperature
Problem 42a
Match the following physical and chemical properties with octane, C8H18 found in gasoline, or magnesium sulfate, MgSO4 also called Epsom salts:
a. contains only covalent bonds
Problem 42d
Match the following physical and chemical properties with octane, C8H18 found in gasoline, or magnesium sulfate, MgSO4 also called Epsom salts:
d. is a liquid at room temperature
Problem 44b
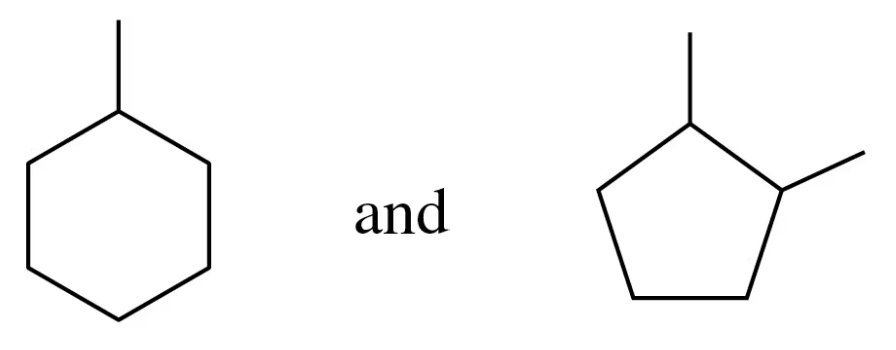
Identify the compounds in each of the following pairs as structural isomers or not structural isomers:
b.
Problem 46a
Convert each of the following line-angle formulas to a condensed structural formula and give its IUPAC name:
b.
Problem 46b
Convert each of the following line-angle formulas to a condensed structural formula and give its IUPAC name:
a.
Problem 47a
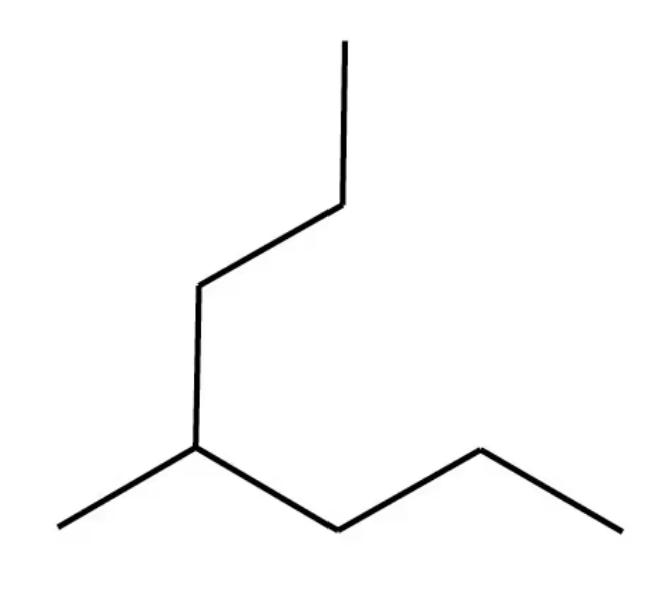
Give the IUPAC name for each of the following:
c.