Back
BackProblem 45d
Complete and balance the equation for each of the following reactions:
d. H2SO4(aq) + Mg(OH)2(s) →
Problem 46a
Complete and balance the equation for each of the following reactions:
c. H2SO4(aq) + Ca(OH)2(s) →
Problem 46b
Complete and balance the equation for each of the following reactions:
b. Ca(s) + H2SO4(aq) →
Problem 47a
Balance each of the following neutralization equations:
a. HCl(aq) + Mg(OH)2(s) → H2O(l) + MgCl2(aq)
Problem 48a
Balance each of the following neutralization equations:
a. HNO3(aq) + Ba(OH)2(s) → H2O(l) + Ba(NO3)2(aq)
Problem 49c
Write a balanced equation for the neutralization of each of the following:
c. HNO3(aq) and Mg(OH)2(s)
Problem 53
If 32.8 mL of a 0.162 M NaOH solution is required to titrate 25.0 mL of a solution of H2SO4, what is the molarity of the H2SO4 solution?
H2SO4(aq) + 2 KOH(aq) → 2 H2O(l) + K2SO4(aq)
Problem 56
Which of the following represents a buffer system? Explain.
a. H3PO3
b. NaNO3
c. HC2H3O2 and NaC2H3O2
d. HCl and NaOH
Problem 62
Someone with severe diabetes obtains energy by the breakdown of fats, which produce large amounts of acidic substances. How would this affect the pH of the blood plasma?
Problem 64
When food enters the stomach, HCl is released and the [H3O+] of the stomach fluid rises to 4 × 10–2 M. What is the pH of the stomach fluid?
Problem 68
Write the balanced chemical equation for the neutralization reaction of stomach acid HCl with Al(OH)3, an ingredient in some antacids.
Problem 70
How many grams of Al(OH)3 are required to neutralize 150. mL of stomach acid with a pH of 1.5?
Problem 71d
Identify each of the following as an acid or a base:
d. HI
Problem 72a
Identify each of the following as an acid or a base:
b. H2SO3
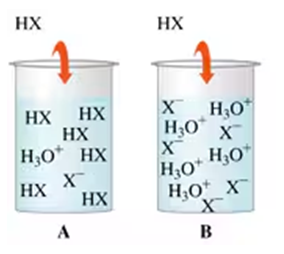
Problem 77
Determine if each of the following diagrams represents a strong acid or a weak acid. The acid has the formula HX.
Problem 79a
Sometimes, during stress or trauma, a person can start to hyperventilate. Then the person might breathe into a paper bag to avoid fainting.
a. What changes occur in the blood pH during hyperventilation?
<IMAGE>
Problem 79b
Sometimes, during stress or trauma, a person can start to hyperventilate. Then the person might breathe into a paper bag to avoid fainting.
b. How does breathing into a paper bag help return blood pH to normal?
<IMAGE>
Problem 81e
Identify each of the following as an acid, base, or salt, and give its name:
e. H2CO3
Problem 82a
Identify each of the following as an acid, base, or salt, and give its name:
a. H3PO4
Problem 88c
Determine the pH for the following solutions:
c. [H3O+] = 0.0001 M
Problem 93a
Solution A has a pH of 4.0, and solution B has a pH of 6.0.
a. Which solution is more acidic?
Problem 94a
Solution X has a pH of 9.0, and solution Y has a pH of 7.0.
a. Which solution is more acidic?
Problem 95
A 0.205 M NaOH solution is used to titrate 20.0 mL of a solution of H2SO4. If 45.6 mL of the NaOH solution is required to reach the endpoint, what is the molarity of the H2SO4 solution?
H2SO4(aq) + 2NaOH(aq) → 2H2O(l) + Na2SO4
Problem 97a
Calculate the volume, in milliliters, of a 0.150 M NaOH solution that will completely neutralize each of the following:
a. 25.0 mL of a 0.288 M HCl solution
Problem 99a
A buffer solution is made by dissolving H3PO4 and NaH2PO4 in water.
a. Write an equation that shows how this buffer neutralizes added acid.
Problem 99b
A buffer solution is made by dissolving H3PO4 and NaH2PO4 in water.
b. Write an equation that shows how this buffer neutralizes added base.
Problem 107a
Determine each of the following for a 0.050 M KOH solution:
a. [H3O+]
Problem 107b
Determine each of the following for a 0.050 M KOH solution:
b. pH
Problem 108a
Determine each of the following for a 0.100 M HBr solution:
a. [H3O+]
Problem 108b
Determine each of the following for a 0.100 M HBr solution:
b. pH