What are isoenzymes?

Trypsin, a peptidase that hydrolyzes polypeptides, functions in the small intestine at an optimum pH of 7.7 to 8.0. How is the rate of a trypsin-catalyzed reaction affected by each of the following conditions?
a. changing the pH to 3.0
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Enzyme Activity and pH
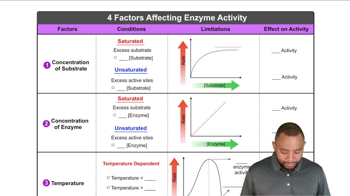
Trypsin Function

Reaction Rate and Environmental Conditions
How is the LDH isoenzyme in the heart different from the LDH isoenzyme in the liver?
A patient arrives in an emergency department complaining of chest pains. What enzymes would you test for in the blood serum?
Trypsin, a peptidase that hydrolyzes polypeptides, functions in the small intestine at an optimum pH of 7.7 to 8.0. How is the rate of a trypsin-catalyzed reaction affected by each of the following conditions?
b. running the reaction at 75 °C
Pepsin, a peptidase that hydrolyzes proteins, functions in the stomach at an optimum pH of 1.5 to 2.0. How is the rate of a pepsin-catalyzed reaction affected by each of the following conditions?
a. changing the pH to 5.0
The following graph shows the activity versus pH curves for pepsin, sucrase, and trypsin. Estimate the optimum pH for each.
<IMAGE>
