The ester methyl butanoate has the odor and flavor of strawberries.
a. Draw the condensed structural formula for methyl butanoate.
<IMAGE>

 Verified step by step guidance
Verified step by step guidance
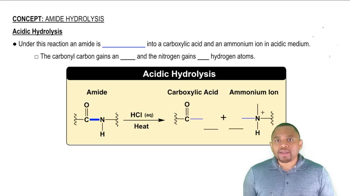
The ester methyl butanoate has the odor and flavor of strawberries.
a. Draw the condensed structural formula for methyl butanoate.
<IMAGE>
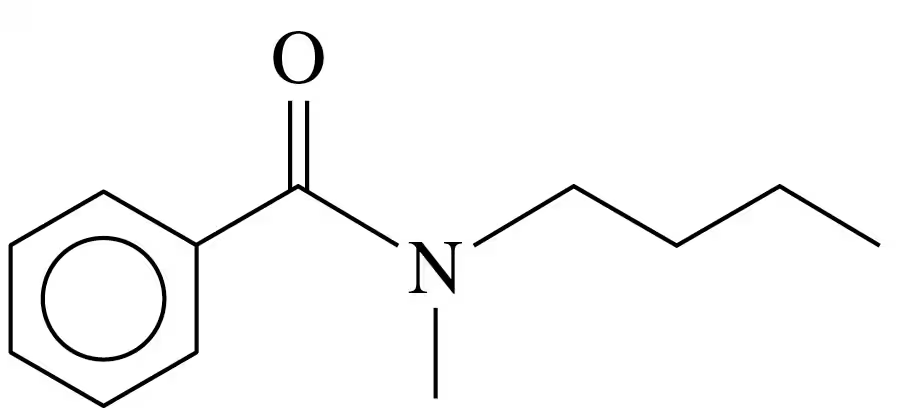
Draw the condensed structural or line-angle formulas for the products from the hydrolysis of each of the following amides with HCl:
b.
Draw the condensed structural or line-angle formulas for the products from the hydrolysis of each of the following amides with HCl:
d.
Draw the condensed structural or line-angle formulas for the products from the hydrolysis of each of the following amides with NaOH:
a.
Draw the condensed structural or line-angle formulas for the products from the hydrolysis of each of the following amides with NaOH:
d.
Draw the condensed structural formulas and write the IUPAC names for two structural isomers of the carboxylic acids that have the molecular formula C4H8O2.