Back
BackProblem 9
Which of the following is found in the coenzyme FAD?
a. Two heterocyclic rings
b. ADP
c. A substituted benzene ring
d. A phosphate anhydride bond
Problem 10a
Look ahead to Figure 21.8 for the citric acid cycle.
<IMAGE>
a. Draw the structures of the reactants in steps 3, 6, and 8, and indicate which hydrogen atoms are removed in these reactions.
Problem 10b
Look ahead to Figure 21.8 for the citric acid cycle.
<IMAGE>
b. What class of enzymes carry out these reactions?
Problem 13
Why, do you suppose, the coenzyme for the reaction in the citric acid cycle that is catalyzed by succinate dehydrogenase is FAD and not NAD+?
Problem 14
Identify the participants in the citric acid cycle that contain alcohol groups. Identify these groups as primary, secondary, or tertiary alcohols.
Problem 15
Which of the reactants in the citric acid cycle have two chiral carbon atoms?
Problem 19
The reduced coenzymes NADH and FADH2 are oxidized in the ETS. What is the final electron acceptor of the ETS? What is the function of the H+ ion in ATP synthesis?
Problem 21a
Each of these reactions is involved in one of the four stages of metabolism shown in Figure 21.4. Identify the stage in which each reaction occurs.
<IMAGE>
a. Hydrolysis of starch to produce glucose
Problem 21b
Each of these reactions is involved in one of the four stages of metabolism shown in Figure 21.4. Identify the stage in which each reaction occurs.
<IMAGE>
b. Oxidation of NADH coupled with synthesis of ATP
Problem 21c
Each of these reactions is involved in one of the four stages of metabolism shown in Figure 21.4. Identify the stage in which each reaction occurs.
<IMAGE>
c. Conversion of glucose to acetyl-CoA
Problem 22
For the first step in fatty acid catabolism, we say that ATP is used to “drive” the reaction that links the fatty acid with coenzyme-A. Without ATP hydrolysis, would you predict that the linking of fatty acid to coenzyme-A would be exergonic or endergonic? In fatty acid CoA synthesis, the hydrolysis of the ATP portion is based on what major strategy of metabolism?
Problem 23
Since no molecular oxygen participates in the citric acid cycle, the steps in which acetyl groups are oxidized to CO2 involve removal of hydride ions and hydrogen ions. What is the acceptor of hydride ions? What is the acceptor of hydrogen ions?
Problem 24a
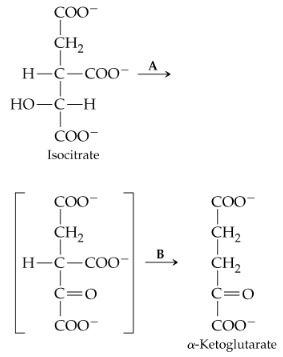
The reaction that follows is catalyzed by isocitrate dehydrogenase and occurs in two steps, the first of which (step A) is formation of an unstable intermediates (shown in brackets).
a. In which step is a coenzyme needed? Identify the coenzyme.
Problem 24b
The reaction that follows is catalyzed by isocitrate dehydrogenase and occurs in two steps, the first of which (step A) is formation of an unstable intermediates (shown in brackets).
b. In which step is CO2 evolved and a hydrogen ion added?
Problem 24c
The reaction that follows is catalyzed by isocitrate dehydrogenase and occurs in two steps, the first of which (step A) is formation of an unstable intermediates (shown in brackets).
c. Which of the structures shown can be described as a β-keto acid?
Problem 24d
The reaction that follows is catalyzed by isocitrate dehydrogenase and occurs in two steps, the first of which (step A) is formation of an unstable intermediates (shown in brackets).
d. To what class of enzymes does isocitrate dehydrogenase, the enzyme that catalyzes this reaction, belong?
Problem 26
The electron-transport chain uses several different metal ions, especially iron, copper, zinc, and manganese. Why are metals used frequently in these two pathways? What can metals do better than organic biomolecules?
Problem 27
What energy requirements must be met in order for a reaction to be favorable?
Problem 29
Why is ∆G a useful quantity for predicting the favorability of biochemical reactions?
Problem 31c
The following reactions occur during the catabolism of acetyl-CoA. Which are exergonic? Which is endergonic? Which reaction produces a phosphate that later yields energy by giving up a phosphate group?
c. L-Malate + NAD+ → Oxaloacetate + NADH + H+
∆G = +17 kcal/mol (+129.3 kJ/mol)
Problem 32b
The following reactions occur during the catabolism of glucose. Which are exergonic? Which is endergonic? Which proceeds farthest toward products at equilibrium?
b. Phosphoenol pyruvate + H2O → Pyruvate + Phosphate(Pi)
∆G = –14.8 kcal/mol (–61.9 kJ/mol)
Problem 39
What is the difference between catabolism and anabolism?
Problem 40
What is the difference between digestion and metabolism?
Problem 41
Arrange the following events in the order in which they occur in a catabolic process: electron transport, digestion, oxidative phosphorylation, citric acid cycle.
Problem 42
What key metabolic intermediate is formed from the catabolism of all three major classes of foods: carbohydrates, lipids, and proteins?
Problem 43
Why is ATP sometimes called a high-energy molecule?
Problem 44
What general kind of chemical reaction does ATP participate in?
Problem 45
What does it mean when we say that two reactions are coupled?
Problem 47
Write the reaction for the hydrolysis of 1,3-bisphosphoglycerate coupled to the phosphorylation of ADP using the curved-arrow symbolism.
Problem 49a
FAD is a coenzyme for dehydrogenation.
a. When a molecule is dehydrogenated, is FAD oxidized or reduced?