Back
BackProblem 53
Reduction of D-fructose with a reducing agent yields a mixture of D-sorbitol along with a second, isomeric product. What is the structure of the second product?
Problem 54
Treatment of an aldose with an oxidizing agent such as Tollens’ reagent yields a carboxylic acid. Gluconic acid, the product of glucose oxidation, is used as its magnesium salt for the treatment of magnesium deficiency. Draw the structure of gluconic acid.
Problem 55
Oxidation of the aldehyde group of ribose yields a carboxylic acid. Draw the structure of ribonic acid.
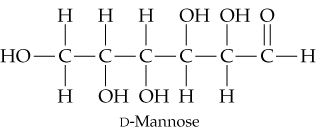
Problem 58
Look at the open-chain form of D-mannose and draw the two glycosidic products that you expect to obtain by reacting D-mannose with methanol.
Problem 59
Draw a disaccharide of two cyclic mannose molecules attached by an α-1,4 glycosidic linkage. Explain why the glycosidic products in Problem 20.58 are not reducing sugars, but the product in this problem is a reducing sugar.
Problem 61
Lactose and maltose are reducing disaccharides, but sucrose is a nonreducing disaccharide. Explain.
Problem 62
Amylose (a form of starch) and cellulose are both polymers of glucose. What is the main structural difference between them? What roles do these two polymers have in nature?
Problem 63
How are amylose and amylopectin similar to each other, and how are they different from each other?
Problem 68
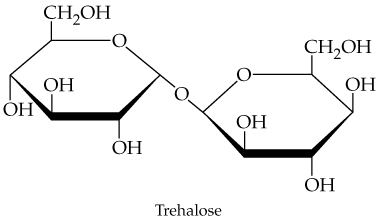
Trehalose, a disaccharide found in the blood of insects, has the following structure. What simple sugars would you obtain on hydrolysis of trehalose? (Hint: Rotate one of the rings in your head or redraw it rotated.)
Problem 75
Are the α and β forms of the disaccharide lactose enantiomers of each other? Why or why not?
Problem 76
D-Fructose can form a six-membered cyclic hemiacetal as well as the more prevalent five-membered cyclic form. Draw the α isomer of D-fructose in the six-membered ring.
Problem 83
Describe the differences between mono-, di-, and polysaccharides.
Problem 84
Name a naturally occurring carbohydrate and its source for each type of carbohydrate listed in Problem 20.83.
Problem 87
Carbohydrates provide 4 kcal per gram. If a person eats 200 g per day of digestible carbohydrates, what percentage of a 2000 kcal daily diet would be digestible carbohydrate?