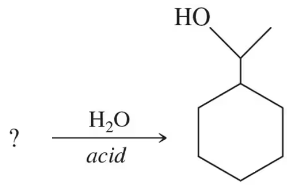
Fill in the missing organic product or reactant for the following hydration reactions:
(b)

 Verified step by step guidance
Verified step by step guidance
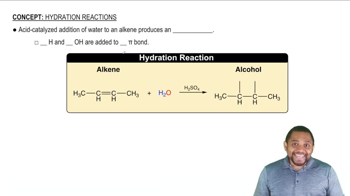
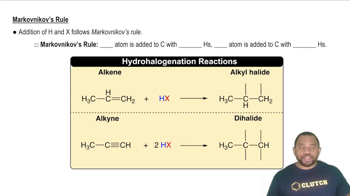
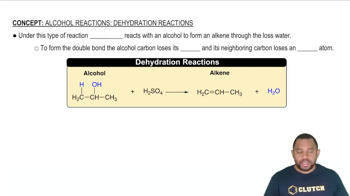
Fill in the missing organic product or reactant for the following hydration reactions:
(b)
Fill in the missing organic products for the complete hydrogenation of the following:
(a)
Fill in the missing organic products for the complete hydrogenation of the following:
(c)
Fill in the missing organic product or reactant for the following hydration reactions:
(b)
How do low-carb diets work? We store glucose molecules in our muscles and liver as glycogen, which consists of thousands of glucose molecules linked together. During periods of fasting, we can activate glycogen to provide glucose.
(a) Determine which of the following reactions below would be a condensation and which would be a hydrolysis.
How do low-carb diets work? We store glucose molecules in our muscles and liver as glycogen, which consists of thousands of glucose molecules linked together. During periods of fasting, we can activate glycogen to provide glucose.
(b) Individuals who do not eat carbohydrates do not store the same levels of glycogen as people who do. Explain the weight loss associated with storing less glycogen.