How would the following changes affect enzyme activity for an enzyme whose optimal conditions are normal body temperature and physiological pH?
a. raising the temperature from 37 °C to 60 °C

 Verified step by step guidance
Verified step by step guidance


How would the following changes affect enzyme activity for an enzyme whose optimal conditions are normal body temperature and physiological pH?
a. raising the temperature from 37 °C to 60 °C
The enzyme urease functions in the body to catalyze the formation of ammonia and carbon dioxide from urea as shown:
Describe what effect the following changes would have on the rate of this reaction assuming a steady state has been reached:
a. adding excess urea
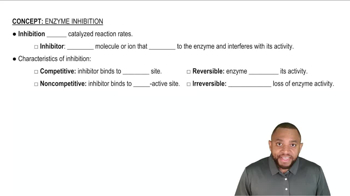
Indicate whether each of the following describes a competitive or a noncompetitive inhibitor.
a. The structure of the inhibitor is similar to that of the substrate.
Indicate whether each of the following describes a competitive or a noncompetitive inhibitor.
e. Adding more substrate to the reaction restores the enzyme activity.
Give the name and three-letter abbreviation for the amino acid described by each of the following:
a. the nonpolar amino acid with a sulfur atom in its side chain
Give the name and three-letter abbreviation for the amino acid described by each of the following:
a. the polar amino acid with a benzene ring in its side chain