8. Gases, Liquids and Solids
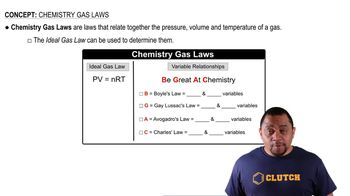
Chemistry Gas Laws
8. Gases, Liquids and Solids
Chemistry Gas Laws
Practice this topic
- Multiple Choice
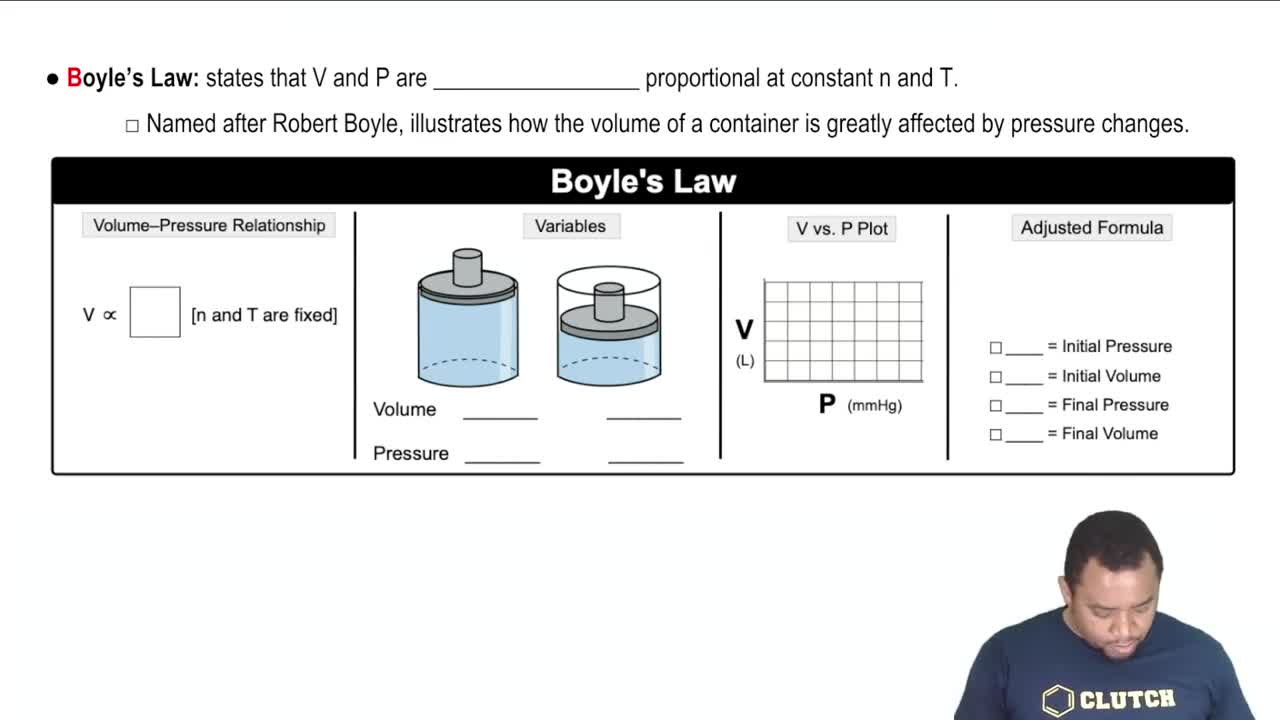
A 10.0 L cylinder with a movable piston exerts 3.00 atm of pressure. What will happen to the pressure if the volume of the container increases to 20.0 L?
a) It will double
b) It will decrease by half
c) It will increase slightly
d) No change
- Multiple Choice
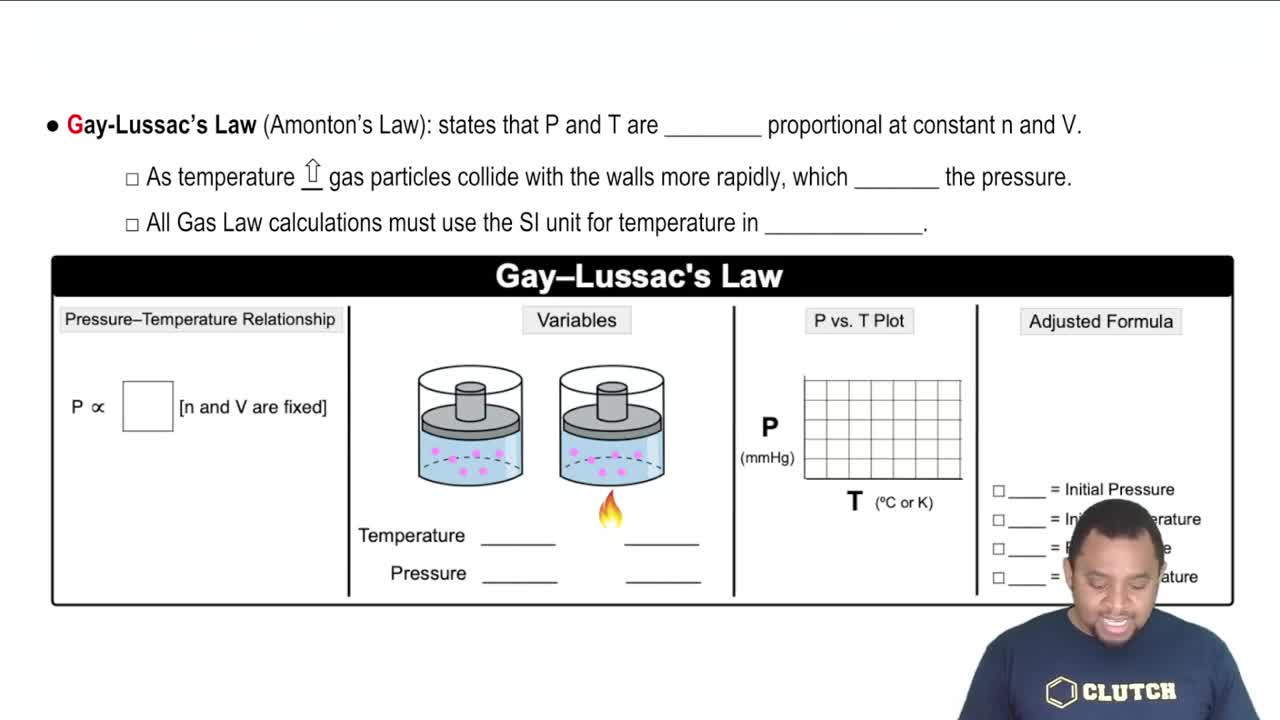
A sealed container with a movable piston contains a gas with a pressure of 1380 torr, a volume of 820 mL and a temperature of 31°C. What would the volume be if the new pressure is now 2.83 atm, while the temperature decreased to 25°C?
- Open QuestionUse the words inspiration and expiration to describe the part of the breathing cycle that occurs as a result of each of the following:a. The diaphragm contracts.
- Open QuestionUse the words inspiration and expiration to describe the part of the breathing cycle that occurs as a result of each of the following:c. The pressure within the lungs is higher than that of the atmosphere.
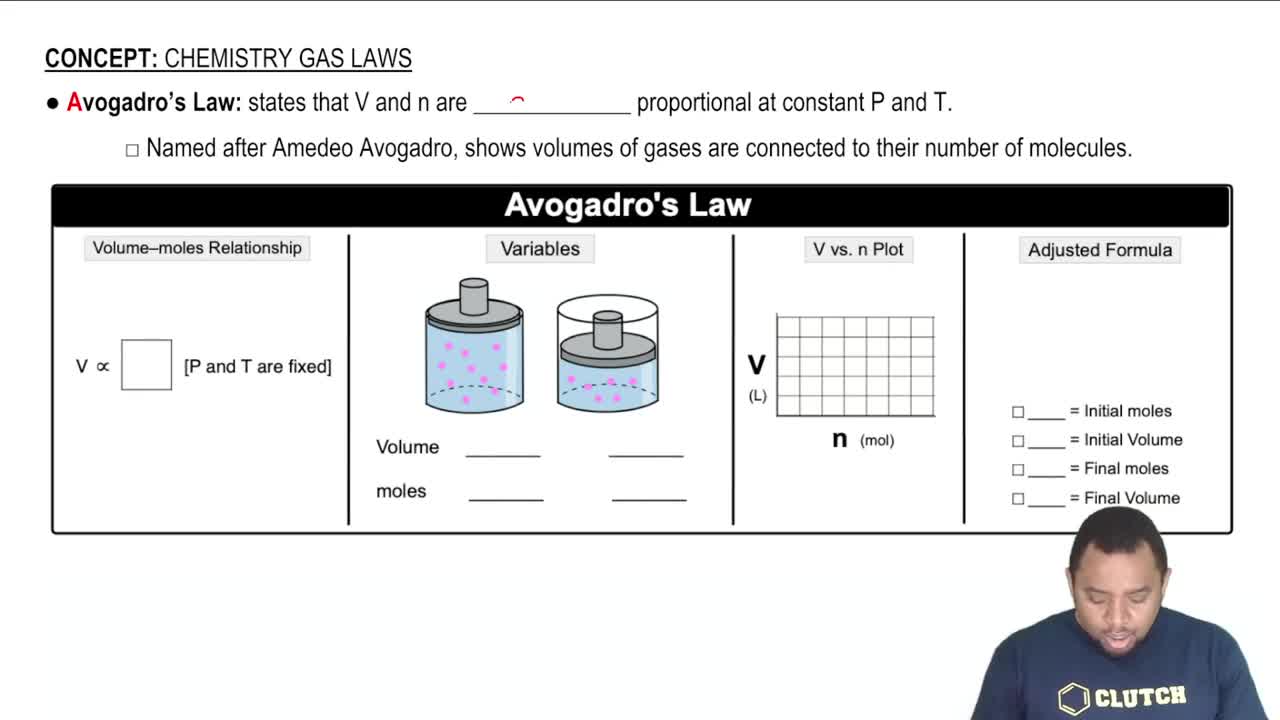
- Open QuestionWhich assumptions of the kinetic–molecular theory explain the behavior of gases described by Boyle's law? Explain your answer.
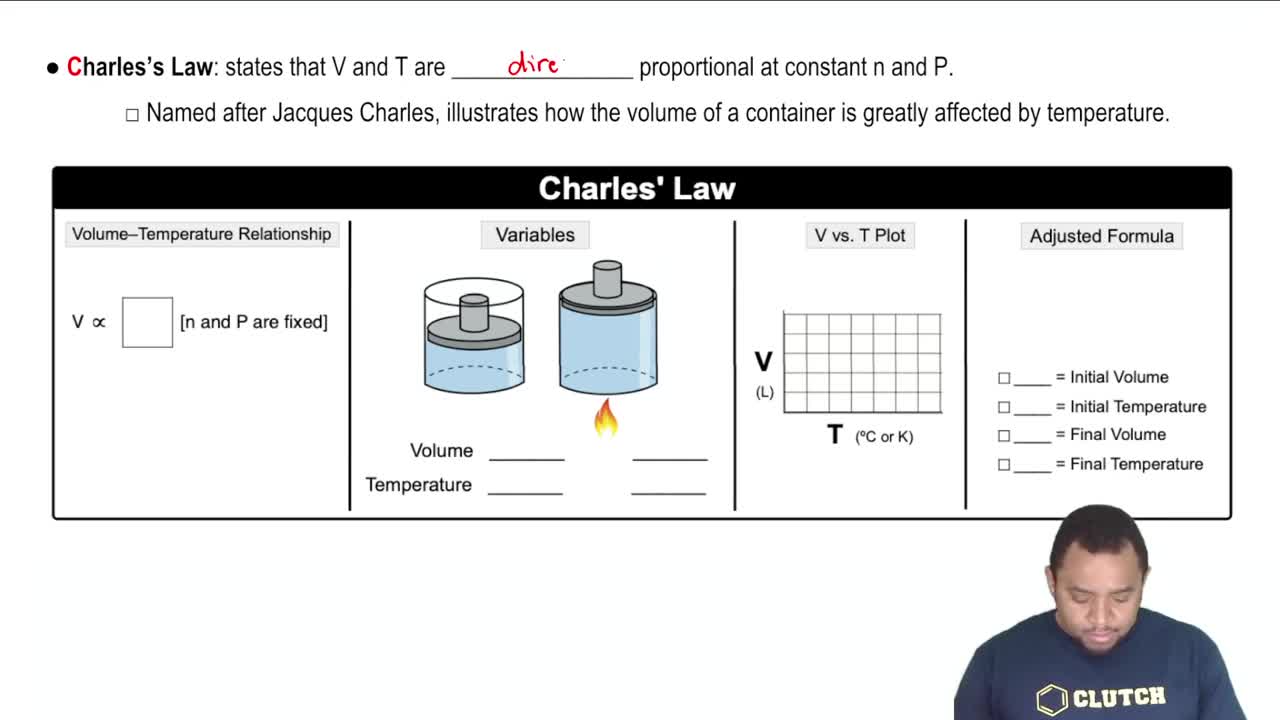
- Open QuestionWhich assumptions of the kinetic–molecular theory explain the behavior of gases described by Charles's law? Explain your answer.