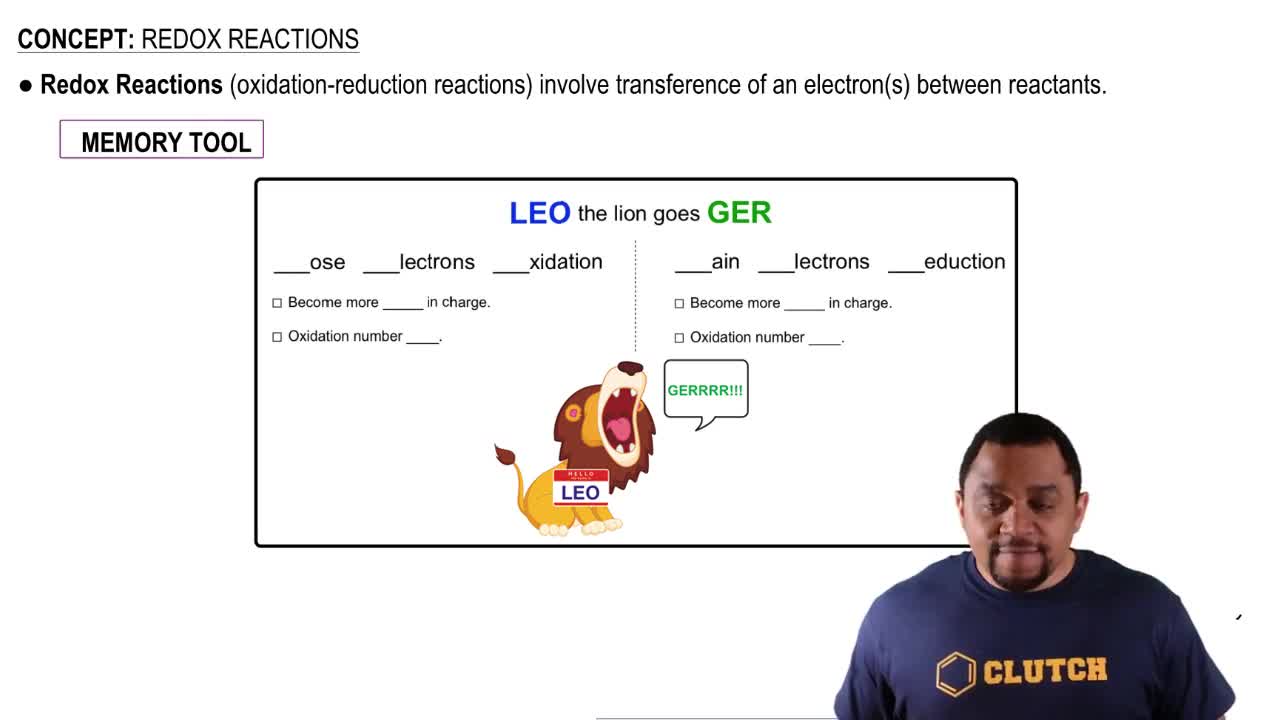
5. Classification & Balancing of Chemical Reactions
Redox Reactions
5. Classification & Balancing of Chemical Reactions
Redox Reactions
Practice this topic
- Multiple Choice
Which element is being reduced in the following reaction?
Cr2O72- + 3 HNO2 + 5 H+ → 2 Cr3+ + 3NO3- + 4 H2O
- Multiple Choice
Identify the oxidizing agent and reducing agent from the following redox reaction.
Ba (s) + Cl2 (g) → BaCl2 (aq)
- Multiple Choice
Which element is oxidized and which is reduced in the following reaction?
Hg (aq) + HgCl2 (aq) → Hg2Cl2
- Multiple Choice
Which of the following represents an oxidation-reduction reaction?
I. PCl3 (aq) + Cl2 (g) → PCl5 (aq)
II. 2 AgNO3 (aq) + Cu (s) → Cu(NO3)2 (aq) + 2 Ag (s)
III. CO2 (g) + 2 LiOH (aq) → Li2CO3 (aq) + H2O (l)
IV. FeCl2 (aq) + 2 NaOH (aq) → Fe(OH)2 (aq) + 2 NaCl (aq)
- Open QuestionIdentify the oxidized reactant, the reduced reactant, the oxidizing agent, and the reducing agent in the following reactions:a. Fe(s)+Cu₂+(aq) → Fe₂+(aq)+Cu(s) b. Mg(s)+Cl₂(g) → MgCl₂(s)c. 2 Al(s)+Cr₂O₃(s) → 2 Cr(s) + Al₂O₃(s)
- Open QuestionPotassium, a silvery metal, reacts with bromine, a corrosive, reddish liquid, to yield potassium bromide, a white solid. Write the balanced equation, and identify the oxidizing and reducing agents.
- Open QuestionIdentify each of the following as an oxidation or a reduction:c. Cr³⁺(aq) + 3e⁻ → Cr(s)
- Open QuestionIn the mitochondria of human cells, energy is provided by the oxidation and reduction reactions of the iron ions in the cytochromes in electron transport. Identify each of the following as an oxidation or a reduction:a. Fe³⁺ + e⁻ → Fe²⁺