11. Nuclear Chemistry
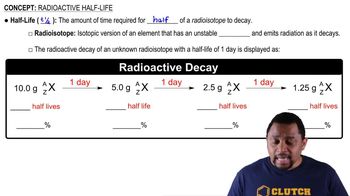
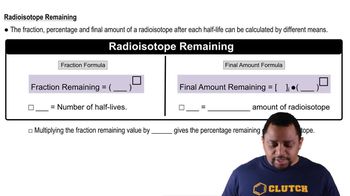
Radioactive Half-Life
11. Nuclear Chemistry
Radioactive Half-Life
Practice this topic
- Multiple Choice
The half-life of arsenic-74 is about 18 days. If a sample initially contains 100.00 mg arsenic-74, what mass (in mg) would be left after 72 days?
- Multiple Choice
The half-life of iodine-131, an isotope used in thyroid therapy, is 8.021 days. What percentage of iodine-131 remains in a sample that is estimated to be 40.11 days old?
- Multiple Choice
What is the concentration of a CO2 in a container after 4 half-lives if 0.325 mol of CO2 is initially placed into a 5.0 L reaction vessel?
- Multiple Choice
What is the half-life of the radioisotope that shows the following plot of remaining percentage vs. time?
- Open QuestionWhat is wrong with the following decay curve? Explain.
- Open QuestionFor each of the following, indicate if the number of half-lives elapsed is:1. one half-life2. two half-lives3. three half-livesc. a sample of Au-198 with a half-life of 2.7 days after 5.4days
- Open QuestionStrontium-85, used for bone scans, has a half-life of 65 days.b. How long will it take for the radiation level of strontium-85 to drop to one-eighth of its original level?
- Open QuestionFluorine-18, which has a half-life of 110 min, is used in PET scans.b. If 100. mg of fluorine-18 is shipped at 8:00 a.m., how many milligrams of the radioisotope are still active when the sample arrives at the radiology laboratory at 1:30p.m.?