A 20.0-mL sample of 0.115 M sulfurous acid (H2SO3) solution is titrated with 0.1014 M KOH. At what added volume of base solution does each equivalence point occur?
Ch.18 - Aqueous Ionic Equilibrium

Chapter 18, Problem 89
Referring to Table 18.1, pick an indicator for use in the titration of each acid with a strong base. a. HF
 Verified step by step guidance
Verified step by step guidance1
Identify the type of titration: In this case, you are titrating a weak acid (HF) with a strong base.
Determine the pH at the equivalence point: For a weak acid-strong base titration, the pH at the equivalence point will be greater than 7.
Consult Table 18.1 for indicators: Look for an indicator that changes color in the pH range that includes the pH at the equivalence point.
Select an appropriate indicator: Choose an indicator whose color change range falls within the expected pH range at the equivalence point.
Verify the choice: Ensure the selected indicator provides a clear and distinct color change at the equivalence point.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
5mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Acid-Base Titration
An acid-base titration is a quantitative analytical method used to determine the concentration of an acid or base in a solution. During the titration, a solution of known concentration (the titrant) is added to a solution of unknown concentration until the reaction reaches its equivalence point, where the amount of acid equals the amount of base. The choice of indicator is crucial as it signals the endpoint of the titration.
Recommended video:
Guided course
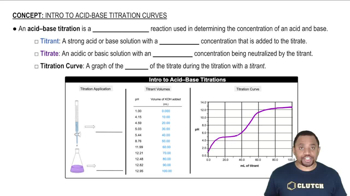
Acid-Base Titration
Indicators
Indicators are substances that change color at a specific pH range, signaling the endpoint of a titration. The choice of indicator depends on the pH at the equivalence point of the titration. For weak acids like HF titrated with a strong base, an indicator that changes color around a pH of 7 to 10 is typically used, as the equivalence point will be above neutral pH due to the formation of a weak conjugate base.
Recommended video:
Guided course
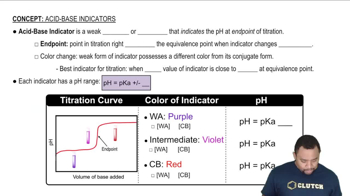
Acid-Base Indicators
HF (Hydrofluoric Acid)
HF is a weak acid that partially dissociates in solution, meaning it does not completely ionize like strong acids. This characteristic affects the pH of the solution during titration and the choice of indicator. When titrating HF with a strong base, the resulting solution will have a higher pH at the equivalence point, necessitating an indicator that can accurately reflect this change.
Recommended video:
Guided course

Binary Acid Identification Example
Related Practice
Textbook Question
Textbook Question
Methyl red has a pKa of 5.0 and is red in its acid form and yellow in its basic form. If several drops of this indicator are placed in a 25.0-mL sample of 0.100 M HCl, what color will the solution appear? If 0.100 M NaOH is slowly added to the HCl sample, in what pH range will the indicator change color?
Textbook Question
Referring to Table 17.1, pick an indicator for use in the titration of each base with a strong acid. a. CH3NH2
Textbook Question
Write balanced equations and expressions for Ksp for the dissolution of each ionic compound. a. BaSO4
Textbook Question
Write balanced equations and expressions for Ksp for the dissolution of each ionic compound. b. PbBr2
