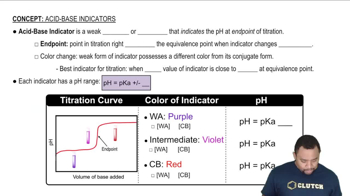
Textbook Question
Methyl red has a pKa of 5.0 and is red in its acid form and yellow in its basic form. If several drops of this indicator are placed in a 25.0-mL sample of 0.100 M HCl, what color will the solution appear? If 0.100 M NaOH is slowly added to the HCl sample, in what pH range will the indicator change color?