Textbook Question
A 0.229-g sample of an unknown monoprotic acid is titrated with 0.112 M NaOH. The resulting titration curve is shown here. Determine the molar mass and pKa of the acid.
1
views

 Verified step by step guidance
Verified step by step guidance
A 0.229-g sample of an unknown monoprotic acid is titrated with 0.112 M NaOH. The resulting titration curve is shown here. Determine the molar mass and pKa of the acid.
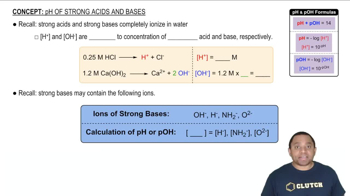
A 20.0-mL sample of 0.115 M sulfurous acid (H2SO3) solution is titrated with 0.1014 M KOH. At what added volume of base solution does each equivalence point occur?
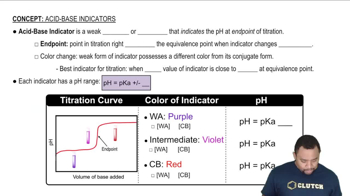
Referring to Table 18.1, pick an indicator for use in the titration of each acid with a strong base. a. HF
Referring to Table 17.1, pick an indicator for use in the titration of each base with a strong acid. a. CH3NH2