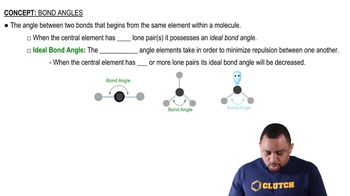
Textbook Question
What shape do you expect for molecules that meet the following descriptions? (a) A central atom with two lone pairs and three bonds to other atoms (b) A central atom with two lone pairs and two bonds to other atoms (c) A central atom with two lone pairs and four bonds to other atoms