Textbook Question
(b) Which has the larger sixth ionization energy, Se or Br?

 McMurry 8th Edition
McMurry 8th Edition Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory
Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory Problem 64
Problem 64 Verified step by step guidance
Verified step by step guidance
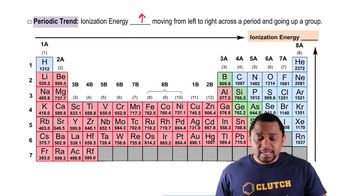

(b) Which has the larger sixth ionization energy, Se or Br?