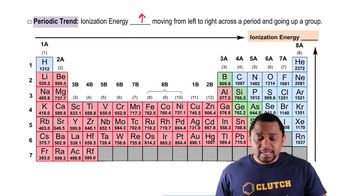
Ionization Energy (Ei)
Ionization energy is the energy required to remove an electron from an atom or ion in its gaseous state. It is a critical concept in understanding how easily an atom can lose electrons, which is influenced by factors such as atomic size, nuclear charge, and electron shielding. Generally, ionization energy increases across a period and decreases down a group in the periodic table.

 McMurry 8th Edition
McMurry 8th Edition Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory
Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory Problem 62
Problem 62 Verified step by step guidance
Verified step by step guidance