Textbook Question
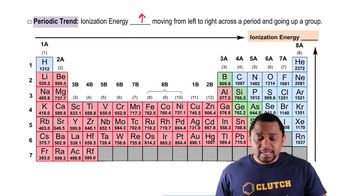
Three atoms have the following electron configurations: (a) 1s2 2s2 2p6 3s2 3p3 (b) 1s2 2s2 2p6 3s2 3p6 (c) 1s2 2s2 2p6 3s2 3p6 4s2 Which of the three has the largest Ei2? Which has the smallest Ei7?

 McMurry 8th Edition
McMurry 8th Edition Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory
Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory Problem 65
Problem 65 Verified step by step guidance
Verified step by step guidance