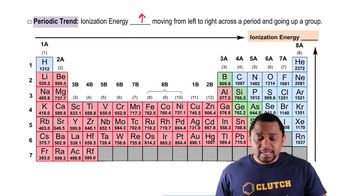
Comparison of Elements
When comparing elements like selenium (Se) and bromine (Br), it is essential to consider their positions in the periodic table. Bromine is to the right of selenium, indicating it has a higher nuclear charge and generally higher ionization energies. However, the sixth ionization energy specifically refers to the energy required to remove the sixth electron, which can be influenced by the electron configuration and stability of the resulting ion.

 McMurry 8th Edition
McMurry 8th Edition Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory
Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory Problem 61b
Problem 61b Verified step by step guidance
Verified step by step guidance