Look at the location of elements A, B, C, and D in the following periodic table:
(a) Write the formula of the simplest binary hydride of each element.

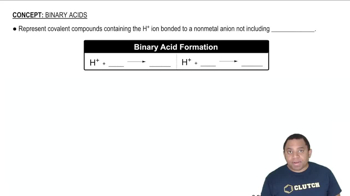
 Verified step by step guidance
Verified step by step guidance


Look at the location of elements A, B, C, and D in the following periodic table:
(a) Write the formula of the simplest binary hydride of each element.
Look at the location of elements A, B, C, and D in the following periodic table:
(d) Which hydrides react with water to give an acidic solution, and which give a basic solution?
Look at the location of elements A, B, C, and D in the following
periodic table:
(c) Which hydrides react with water to give H2 gas? Write a balanced net ionic equation for each reaction.
Which of the following oxides will be more soluble in acidic solution? (LO 22.14)
(a) SiO2 (b) NO2
(c) BaO (d) B2O3
In what forms is oxygen commonly found in nature?
Explain why the properties of boron differ so markedly from the properties of the other group 3A elements.