Textbook Question
Look at the location of elements A, B, C, and D in the following periodic table:
(d) Which hydrides react with water to give an acidic solution, and which give a basic solution?

 Verified step by step guidance
Verified step by step guidance
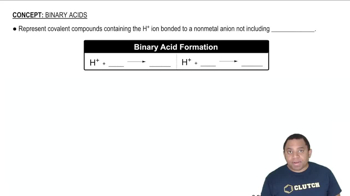


Look at the location of elements A, B, C, and D in the following periodic table:
(d) Which hydrides react with water to give an acidic solution, and which give a basic solution?
Look at the location of elements A, B, C, and D in the following
periodic table:
(c) Which hydrides react with water to give H2 gas? Write a balanced net ionic equation for each reaction.
The following models represent the structures of binary hydrides
of second-row elements:
(a) Identify the nonhydrogen atom in each case, and write the molecular formula for each hydride.