For each of the following complexes, draw a crystal field energy-level diagram, assign the electrons to orbitals, and predict the number of unpaired electrons.
(c) [Co(NCS)4]2- (tetrahedral)

 McMurry 8th Edition
McMurry 8th Edition Ch.21 - Transition Elements and Coordination Chemistry
Ch.21 - Transition Elements and Coordination Chemistry Problem 21.140d
Problem 21.140d Verified step by step guidance
Verified step by step guidance


For each of the following complexes, draw a crystal field energy-level diagram, assign the electrons to orbitals, and predict the number of unpaired electrons.
(c) [Co(NCS)4]2- (tetrahedral)
Nickel(II) complexes with the formula NiX2L2, where X is Cl- or N-bonded NCS- and L is the monodentate triphenylphosphine ligand P(C6H5)3, can be square planar or tetrahedral.
(c) Draw possible structures for each of the NiX2L2 complexes, and tell which ones have a dipole moment.
Spinach contains a lot of iron but is not a good source of dietary iron because nearly all the iron is tied up in the oxalate complex [Fe(C2O4)3]3-.
(d) Draw the structure of [Fe(C2O4)3]3-. Is the complex chiral or achiral?
In acidic aqueous solution, the complex trans-[Co(en)2Cl1]2+(aq) undergoes the following substitution reaction:
trans-[Co(en)1Cl2]+(aq) + H2O(l) → trans-[Co(en)2(H2O)Cl]2+(aq) + Cl–(aq)
The reaction is first order in trans-[Co(en)2Cl2]+(aq), and the rate constant at 25°C is 3.2×10–5 s–1.
(d) Is the reaction product chiral or achiral? Explain.
In acidic aqueous solution, the complex trans-[Co(en)2Cl1]2+(aq) undergoes the following substitution reaction:
trans-[Co(en)2Cl1]+(aq) + H2O(l) → trans-[Co(en)2(H2O)Cl]2+(aq) + Cl–(aq)
The reaction is first order in trans-[Co(en)2Cl2]+(aq), and the rate constant at 25°C is 3.2×10–5 s–1.
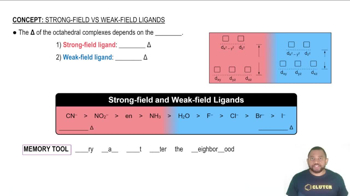
e. Draw a crystal field energy-level diagram for trans-[Co(en)2Cl2]+ that takes account of the fact that Cl– is a weaker-field ligand than ethylenediamine.
The complete reaction of 2.60 g of chromium metal with 50.00 mL of 1.200 M H2SO4 in the absence of air gave a blue solution and a colorless gas that was collected at 25°C and a pressure of 735 mm Hg. (e) When an excess of KCN is added to the solution, the color changes, and the paramagnetism of the solution
decreases. Explain.