Spinach contains a lot of iron but is not a good source of dietary iron because nearly all the iron is tied up in the oxalate complex [Fe(C2O4)3]3-.
(d) Draw the structure of [Fe(C2O4)3]3-. Is the complex chiral or achiral?

 McMurry 8th Edition
McMurry 8th Edition Ch.21 - Transition Elements and Coordination Chemistry
Ch.21 - Transition Elements and Coordination Chemistry Problem 21.138e
Problem 21.138e Verified step by step guidance
Verified step by step guidance

Spinach contains a lot of iron but is not a good source of dietary iron because nearly all the iron is tied up in the oxalate complex [Fe(C2O4)3]3-.
(d) Draw the structure of [Fe(C2O4)3]3-. Is the complex chiral or achiral?
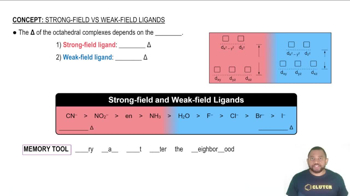
Cobalt(III) trifluoroacetylacetonate, Co1tfac23, is a sixcoordinate, octahedral metal chelate in which three planar, bidentate tfac ligands are attached to a central Co atom:
(d) Draw a crystal field energy-level diagram for Co1tfac23, and predict its magnetic properties. (In this complex, tfac is a strong-field ligand.)
In acidic aqueous solution, the complex trans-[Co(en)2Cl1]2+(aq) undergoes the following substitution reaction:
trans-[Co(en)1Cl2]+(aq) + H2O(l) → trans-[Co(en)2(H2O)Cl]2+(aq) + Cl–(aq)
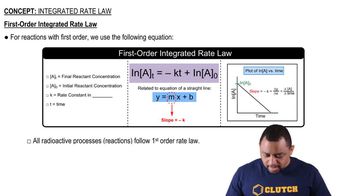
The reaction is first order in trans-[Co(en)2Cl2]+(aq), and the rate constant at 25°C is 3.2×10–5 s–1.
(d) Is the reaction product chiral or achiral? Explain.
The complete reaction of 2.60 g of chromium metal with 50.00 mL of 1.200 M H2SO4 in the absence of air gave a blue solution and a colorless gas that was collected at 25°C and a pressure of 735 mm Hg. (e) When an excess of KCN is added to the solution, the color changes, and the paramagnetism of the solution
decreases. Explain.
Locate on the periodic table the transition elements with the following electron configurations. Identify each element.
(a) [Ar] 3d74s2
(b) [Ar] 3d54s1
Locate on the periodic table the transition elements with the following electron configurations. Identify each element.
(c) [Kr] 4d25s2
(d) [Xe] 4f36s2