Textbook Question
What conditions must be met for a cell potential E to qualify as a standard cell potential E°?
3
views

 Verified step by step guidance
Verified step by step guidance


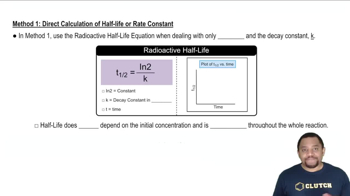
Consider the following substances: Fe(s), PbO2(s), H+(aq), Al(s), Ag(s), Cr2O72-(aq). (a) Look at the E° values in Appendix D, and classify each substance as an oxidizing agent or a reducing agent.