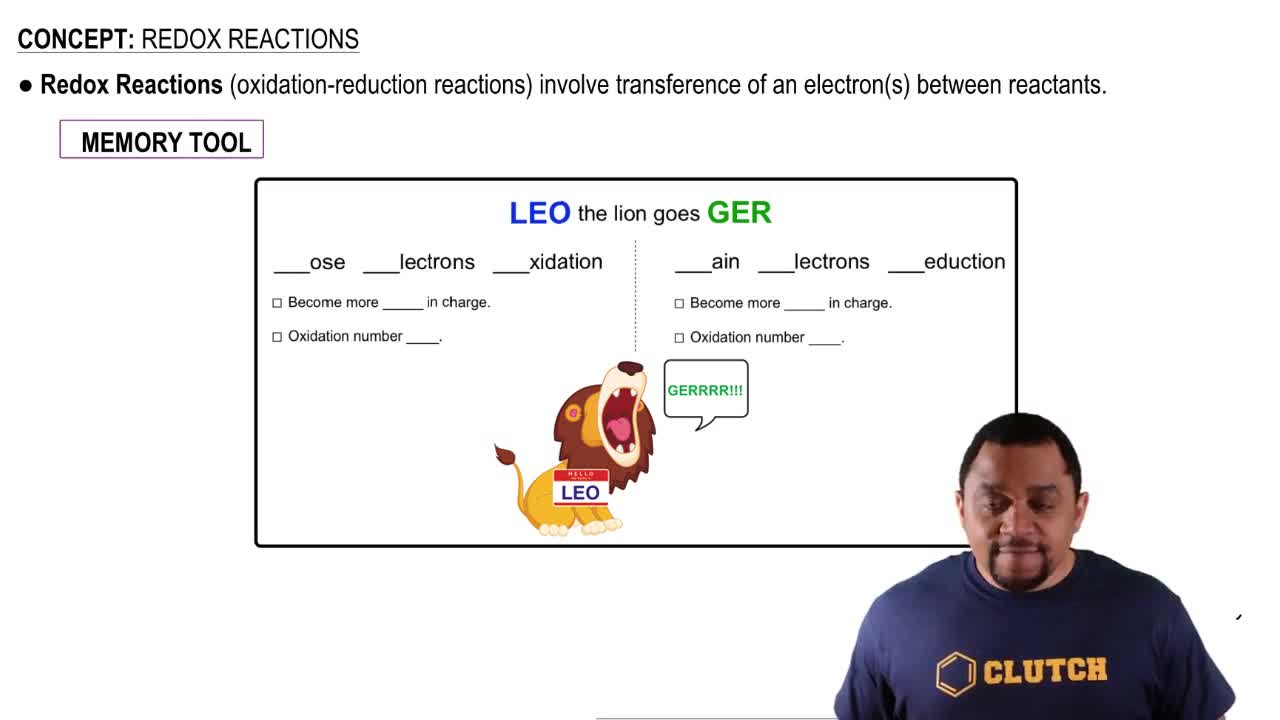
Textbook Question
Consider the following substances: I2(s), Fe2+(aq), Cr2O72-(aq). Which is the strongest oxidizing agent? Which is the weakest oxidizing agent?

 Verified step by step guidance
Verified step by step guidance

Consider the following substances: Fe(s), PbO2(s), H+(aq), Al(s), Ag(s), Cr2O72-(aq). (a) Look at the E° values in Appendix D, and classify each substance as an oxidizing agent or a reducing agent.
The following cell reactions occur spontaneously: (a) Arrange the following reduction half-reactions in order of decreasing tendency to occur: