Textbook Question
For each of these contour representations of molecular orbitals, identify (a) the atomic orbitals (s or p) used to construct the MO (i)


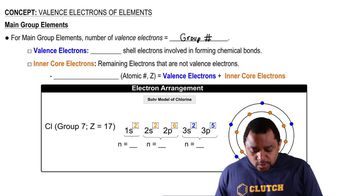
 Verified step by step guidance
Verified step by step guidance


For each of these contour representations of molecular orbitals, identify (a) the atomic orbitals (s or p) used to construct the MO (i)
For each of these contour representations of molecular orbitals, identify (b) the type of MO (s or p) (iii)
For each of these contour representations of molecular orbitals, identify (b) the type of MO (s or p) (i)
a. Methane (CH4) and the perchlorate ion (ClO4−) are both described as tetrahedral. What does this indicate about their bond angles?
b. The NH3 molecule is trigonal pyramidal, while BF3 is trigonal planar. Which of these molecules is flat?
Describe the bond angles to be found in each of the following molecular structures: (a) trigonal planar, (b) tetrahedral, (c) octahedral, (d) linear.