Textbook Question
For each of these contour representations of molecular orbitals, identify (c) whether the MO is bonding or antibonding (i)

 Verified step by step guidance
Verified step by step guidance


For each of these contour representations of molecular orbitals, identify (c) whether the MO is bonding or antibonding (i)
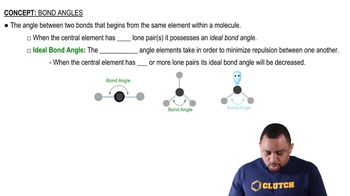
a. Methane (CH4) and the perchlorate ion (ClO4−) are both described as tetrahedral. What does this indicate about their bond angles?
b. The NH3 molecule is trigonal pyramidal, while BF3 is trigonal planar. Which of these molecules is flat?
(b) An AB4 molecule has two lone pairs of electrons on the A atom (in addition to the four B atoms). What is the electron-domain geometry around the A atom?
Would you expect the nonbonding electron-pair domain in NH3 to be greater or less in size than the corresponding one in PH3?