Textbook Question
Assume that you encounter the following sentences in your reading. What is the chemical formula for each substance mentioned? (c) Hydrogen cyanide is a very poisonous gas.

 Verified step by step guidance
Verified step by step guidance
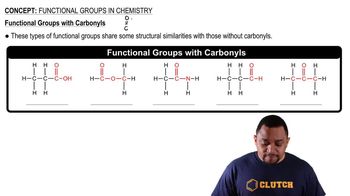
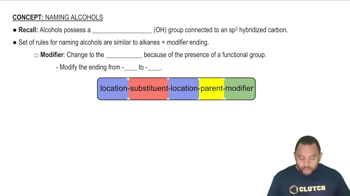
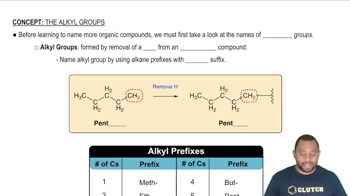
Assume that you encounter the following sentences in your reading. What is the chemical formula for each substance mentioned? (c) Hydrogen cyanide is a very poisonous gas.
a. What elements are contained in hydrocarbons?
d. What is the empirical formula for octane?
b. Write a structural formula for 1-pentanol.
All the structures shown here have the molecular formula C8H18. Which structures are the same molecule? (Hint: One way to answer this question is to determine the chemical name for each structure.)