Write the chemical formula for each substance mentioned in the following word descriptions (use the front inside cover to find the symbols for the elements you do not know). (b) On treatment with hydrofluoric acid, silicon dioxide forms silicon tetrafluoride and water. (use the front inside cover to find the symbols for the elements you do not know). (c) Sulfur dioxide reacts with water to form sulfurous acid. (use the front inside cover to find the symbols for the elements you do not know). (d) The substance phosphorus trihydride, commonly called phosphine, is a toxic gas. (e) Perchloric acid reacts with cadmium to form cadmium(II) perchlorate.
Ch.2 - Atoms, Molecules, and Ions

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 2, Problem 81a
a. What elements are contained in hydrocarbons?
 Verified step by step guidance
Verified step by step guidance1
Identify the definition of hydrocarbons: Hydrocarbons are organic compounds consisting entirely of hydrogen and carbon atoms.
Recognize that hydrocarbons are the simplest form of organic compounds, forming the basis for more complex molecules.
Understand that the carbon atoms in hydrocarbons can be arranged in various structures, such as chains, rings, or branches.
Note that hydrocarbons can be classified into different types, such as alkanes, alkenes, and alkynes, based on the types of bonds between carbon atoms.
Conclude that the elements contained in hydrocarbons are exclusively carbon (C) and hydrogen (H).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
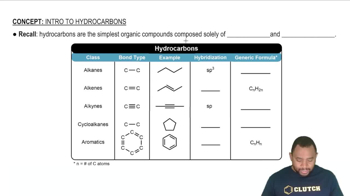
Hydrocarbons
Hydrocarbons are organic compounds composed exclusively of hydrogen and carbon atoms. They serve as the fundamental building blocks of organic chemistry and can be classified into various categories, including alkanes, alkenes, and alkynes, based on the types of bonds between carbon atoms.
Recommended video:
Guided course
Intro To Hydrocarbons
Types of Hydrocarbons
Hydrocarbons can be categorized into two main types: saturated and unsaturated. Saturated hydrocarbons, like alkanes, contain only single bonds between carbon atoms, while unsaturated hydrocarbons, such as alkenes and alkynes, contain one or more double or triple bonds, respectively, affecting their reactivity and properties.
Recommended video:
Guided course

Intro To Hydrocarbons
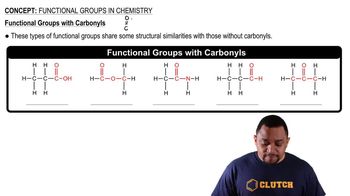
Functional Groups
While hydrocarbons consist solely of carbon and hydrogen, they can also serve as the backbone for more complex molecules that contain functional groups. These groups, such as hydroxyl (-OH) or carboxyl (-COOH), can significantly alter the chemical behavior and properties of the hydrocarbon, leading to a diverse range of organic compounds.
Recommended video:
Guided course
Carbonyl Functional Groups
Related Practice
Textbook Question
Textbook Question
Assume that you encounter the following sentences in your reading. What is the chemical formula for each substance mentioned? (c) Hydrogen cyanide is a very poisonous gas.
Textbook Question
d. What is the empirical formula for octane?
Textbook Question
a. What functional group characterizes an alcohol?
Textbook Question
b. Write a structural formula for 1-pentanol.
