(a) Calculate the pH of a buffer that is 0.12 M in lactic acid and 0.11 M in sodium lactate.
Ch.17 - Additional Aspects of Aqueous Equilibria

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 17, Problem 23
A buffer is prepared by adding 20.0 g of sodium acetate (CH3COONa) to 500 mL of a 0.150 M acetic acid (CH3COOH) solution. (b) Write the complete ionic equation for the reaction that occurs when a few drops of hydrochloric acid are added to the buffer. (c) Write the complete ionic equation for the reaction that occurs when a few drops of sodium hydroxide solution are added to the buffer.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Identify the components of the buffer solution. The buffer is composed of acetic acid (CH3COOH) and its conjugate base, acetate ion (CH3COO^-), from sodium acetate (CH3COONa).
Step 2: Consider the addition of hydrochloric acid (HCl) to the buffer. HCl is a strong acid that will dissociate completely in solution to form H^+ and Cl^- ions.
Step 3: Write the complete ionic equation for the reaction with HCl. The H^+ ions from HCl will react with the acetate ions (CH3COO^-) in the buffer to form acetic acid (CH3COOH). The equation is: CH3COO^- (aq) + H^+ (aq) -> CH3COOH (aq).
Step 4: Consider the addition of sodium hydroxide (NaOH) to the buffer. NaOH is a strong base that will dissociate completely in solution to form Na^+ and OH^- ions.
Step 5: Write the complete ionic equation for the reaction with NaOH. The OH^- ions from NaOH will react with the acetic acid (CH3COOH) in the buffer to form water (H2O) and acetate ions (CH3COO^-). The equation is: CH3COOH (aq) + OH^- (aq) -> CH3COO^- (aq) + H2O (l).
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Buffer Solutions
A buffer solution is a system that resists changes in pH upon the addition of small amounts of acid or base. It typically consists of a weak acid and its conjugate base, or a weak base and its conjugate acid. In this case, acetic acid (CH3COOH) and sodium acetate (CH3COONa) form a buffer that helps maintain a stable pH when hydrochloric acid or sodium hydroxide is introduced.
Recommended video:
Guided course
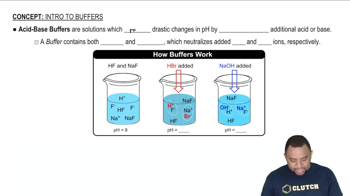
Buffer Solutions
Ionic Equations
Ionic equations represent the species that are actually involved in a chemical reaction, showing the ions in their dissociated form. In the context of buffer reactions, writing complete ionic equations helps illustrate how the buffer components react with added acids or bases. This is crucial for understanding how the buffer maintains pH by neutralizing the added substances.
Recommended video:
Guided course

Net Ionic Equations
Acid-Base Reactions
Acid-base reactions involve the transfer of protons (H+) between reactants. In the case of the buffer, when hydrochloric acid (HCl) is added, it donates protons to the acetate ions (CH3COO-) from sodium acetate, while sodium hydroxide (NaOH) donates hydroxide ions (OH-) that react with acetic acid (CH3COOH). Understanding these reactions is essential for predicting how the buffer will respond to changes in pH.
Recommended video:
Guided course
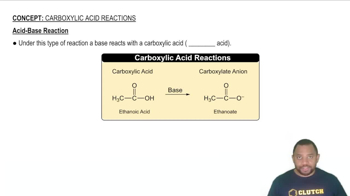
Acid-Base Reaction
Related Practice
Textbook Question
Textbook Question
(b) Calculate the pH of a buffer formed by mixing 85 mL of 0.13 M lactic acid with 95 mL of 0.15 M sodium lactate.
Textbook Question
You are asked to prepare a pH = 3.00 buffer solution starting from 1.25 L of a 1.00 M solution of hydrofluoric acid (HF) and any amount you need of sodium fluoride (NaF). (a) What is the pH of the hydrofluoric acid solution prior to adding sodium fluoride?
Textbook Question
You are asked to prepare a pH = 3.00 buffer solution starting from 1.25 L of a 1.00 M solution of hydrofluoric acid (HF) and any amount you need of sodium fluoride (NaF). (b) How many grams of sodium fluoride should be added to prepare the buffer solution? Neglect the small volume change that occurs when the sodium fluoride is added.
