Which of the following solutions is a buffer? (a) A solution made by mixing 100 mL of 0.100 M CH3COOH and 50 mL of 0.100 M NaOH, (b) a solution made by mixing 100 mL of 0.100 M CH3COOH and 500 mL of 0.100 M NaOH, (c) A solution made by mixing 100 mL of 0.100 M CH3COOH and 50 mL of 0.100 M HCl, (d) A solution made by mixing 100 mL of 0.100 M CH3COOK and 50 mL of 0.100 M KCl.
Ch.17 - Additional Aspects of Aqueous Equilibria

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 17, Problem 22
(a) Calculate the pH of a buffer that is 0.105 M in NaHCO3 and 0.125 M in Na2CO3. (b) Calculate the pH of a solution formed by mixing 65 mL of 0.20 M NaHCO3 with 75 mL of 0.15 M Na2CO3.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Identify the components of the buffer system. NaHCO3 (sodium bicarbonate) acts as the weak acid (HCO3^-), and Na2CO3 (sodium carbonate) acts as the conjugate base (CO3^2-).
Step 2: Use the Henderson-Hasselbalch equation to calculate the pH of the buffer: \( \text{pH} = \text{pK}_a + \log \left( \frac{[\text{Base}]}{[\text{Acid}]} \right) \). Find the \( \text{pK}_a \) value for HCO3^-.
Step 3: For part (a), plug in the concentrations of NaHCO3 and Na2CO3 into the Henderson-Hasselbalch equation: \( [\text{Acid}] = 0.105 \text{ M} \) and \( [\text{Base}] = 0.125 \text{ M} \).
Step 4: For part (b), calculate the moles of NaHCO3 and Na2CO3 in the mixed solution. Use the formula: \( \text{moles} = \text{concentration} \times \text{volume} \). Convert volumes from mL to L.
Step 5: Determine the new concentrations of NaHCO3 and Na2CO3 in the total volume of the mixed solution. Use these concentrations in the Henderson-Hasselbalch equation to find the pH.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Buffer Solutions
Buffer solutions are mixtures that resist changes in pH when small amounts of acid or base are added. They typically consist of a weak acid and its conjugate base or a weak base and its conjugate acid. In this case, NaHCO3 (sodium bicarbonate) acts as the weak acid, while Na2CO3 (sodium carbonate) serves as its conjugate base, allowing the solution to maintain a stable pH.
Recommended video:
Guided course
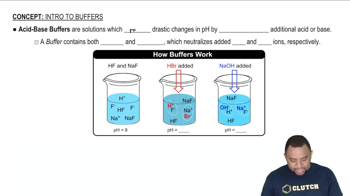
Buffer Solutions
Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation is a mathematical formula used to calculate the pH of buffer solutions. It is expressed as pH = pKa + log([A-]/[HA]), where pKa is the negative logarithm of the acid dissociation constant, [A-] is the concentration of the conjugate base, and [HA] is the concentration of the weak acid. This equation is essential for determining the pH of the buffer in the given question.
Recommended video:
Guided course
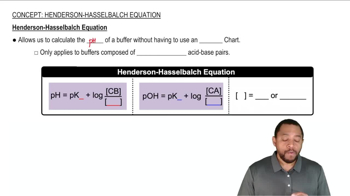
Henderson-Hasselbalch Equation
Dilution and Concentration Calculations
Dilution involves reducing the concentration of a solute in a solution, typically by adding more solvent. The concentration of a solution can be calculated using the formula C1V1 = C2V2, where C1 and V1 are the initial concentration and volume, and C2 and V2 are the final concentration and volume. This concept is crucial for calculating the pH of the mixed solution in part (b) of the question.
Recommended video:
Guided course

Dilution Calculation Example
Related Practice
Textbook Question
2
views
Textbook Question
(a) Calculate the pH of a buffer that is 0.12 M in lactic acid and 0.11 M in sodium lactate.
Textbook Question
(b) Calculate the pH of a buffer formed by mixing 85 mL of 0.13 M lactic acid with 95 mL of 0.15 M sodium lactate.
Textbook Question
You are asked to prepare a pH = 3.00 buffer solution starting from 1.25 L of a 1.00 M solution of hydrofluoric acid (HF) and any amount you need of sodium fluoride (NaF). (a) What is the pH of the hydrofluoric acid solution prior to adding sodium fluoride?
