(b) Imagine that you could hold two atoms that are bonded together, twist them, and not change the bond length. Would it be easier to twist (rotate) around a single s bond or around a double 1s plus p2 bond, or would they be the same?
Ch.9 - Molecular Geometry and Bonding Theories

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 9, Problem 58
The oxygen atoms in O2 participate in multiple bonding, whereas those in hydrogen peroxide, H2O2, do not. What is the hybridization of the oxygen atoms in each molecule? What is the hybridization of the oxygen atoms in O2? What is the hybridization of the oxygen atoms in H2O2?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concept of hybridization, which involves the mixing of atomic orbitals to form new hybrid orbitals suitable for the pairing of electrons to form chemical bonds.
Step 2: Analyze the molecular structure of O2. Oxygen in O2 forms a double bond, which involves one sigma (σ) bond and one pi (π) bond. The hybridization of oxygen in O2 is determined by the sigma bond.
Step 3: Determine the hybridization of oxygen in O2. Since the oxygen atom forms one sigma bond and has two lone pairs, it uses sp2 hybridization, which involves one s orbital and two p orbitals.
Step 4: Examine the molecular structure of H2O2. Each oxygen atom in H2O2 forms two sigma bonds (one with hydrogen and one with the other oxygen) and has two lone pairs.
Step 5: Determine the hybridization of oxygen in H2O2. The oxygen atoms in H2O2 use sp3 hybridization, which involves one s orbital and three p orbitals, to accommodate the two sigma bonds and two lone pairs.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Hybridization
Hybridization is a concept in chemistry that describes the mixing of atomic orbitals to form new hybrid orbitals, which can explain the geometry and bonding properties of molecules. In hybridization, atomic orbitals such as s, p, and sometimes d orbitals combine to create equivalent orbitals that are oriented in specific directions to minimize electron repulsion.
Recommended video:
Guided course
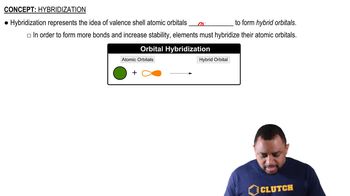
Hybridization
Molecular Geometry
Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule. The geometry is determined by the number of bonding pairs and lone pairs of electrons around the central atom, which influences the hybridization state. For example, in O2, the linear arrangement corresponds to sp hybridization, while in H2O2, the bent structure indicates a different hybridization.
Recommended video:
Guided course
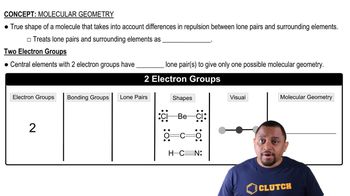
Molecular Geometry with Two Electron Groups
Bonding Types
Bonding types, including single, double, and triple bonds, describe how atoms are connected in a molecule. In O2, the oxygen atoms are connected by a double bond, which involves the sharing of two pairs of electrons, leading to sp hybridization. In contrast, H2O2 has single bonds between oxygen and hydrogen, resulting in a different hybridization state for the oxygen atoms, typically sp3.
Recommended video:
Guided course
Bonding Types
Related Practice
Textbook Question
Textbook Question
(a) Draw Lewis structures for chloromethane (CH3Cl), chloroethene (C2H3Cl), and chloroethyne (C2HCl).
Textbook Question
(b) What is the hybridization of the carbon atoms in each molecule?
1
views
Textbook Question
Propylene, C3H6, is a gas that is used to form the important polymer called polypropylene. Its Lewis structure is (a) What is the total number of valence electrons in the propylene molecule?
Textbook Question
Benzaldehyde, C7H6O, is a fragrant substance responsible for the aroma of almonds. Its Lewis structure is
(a) What is the hybridization at each of the carbon atoms of the molecule?
Textbook Question
Ethyl acetate, C4H8O2, is a fragrant substance used both as a solvent and as an aroma enhancer. Its Lewis structure is
(c) How many of the valence electrons are used to make s bonds in the molecule?
