In the following pairs of binary compounds, determine which one is a molecular substance and which one is an ionic substance. Use the appropriate naming convention (for ionic or molecular substances) to assign a name to each compound: (a) TiCl4 and CaF2 (b) ClF3 and VF3
Ch.8 - Basic Concepts of Chemical Bonding

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 8, Problem 46c
In the following pairs of binary compounds, determine which one is a molecular substance and which one is an ionic substance. Use the appropriate naming convention (for ionic or molecular substances) to assign a name to each compound: (c) SbCl5 and AlF3.
 Verified step by step guidance
Verified step by step guidance1
Determine the type of elements involved in each compound. Molecular compounds typically consist of nonmetals, while ionic compounds consist of metals and nonmetals.
For SbCl_5, identify the elements: Sb (antimony) is a metalloid and Cl (chlorine) is a nonmetal.
For AlF_3, identify the elements: Al (aluminum) is a metal and F (fluorine) is a nonmetal.
Classify SbCl_5 as a molecular compound because it involves a metalloid and nonmetals.
Classify AlF_3 as an ionic compound because it involves a metal and nonmetals.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ionic vs. Molecular Compounds
Ionic compounds are formed from the electrostatic attraction between positively and negatively charged ions, typically between metals and nonmetals. In contrast, molecular compounds consist of molecules formed by covalent bonds, where atoms share electrons, usually between nonmetals. Understanding the distinction helps in identifying the nature of the compounds in question.
Recommended video:
Guided course

Ionic Compounds Naming
Naming Ionic Compounds
Ionic compounds are named by stating the name of the cation (positive ion) followed by the name of the anion (negative ion). For example, in AlF3, aluminum (Al) is the cation and fluoride (F) is the anion, resulting in the name aluminum fluoride. This naming convention is essential for correctly identifying and communicating the nature of ionic substances.
Recommended video:
Guided course

Ionic Compounds Naming
Naming Molecular Compounds
Molecular compounds are named using prefixes to indicate the number of atoms of each element present in the molecule. For instance, in SbCl5, antimony (Sb) is the central atom with five chlorine (Cl) atoms, leading to the name pentachloride antimony. This systematic approach to naming helps distinguish molecular compounds from ionic ones.
Recommended video:
Guided course
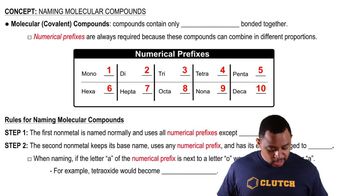
Naming Molecular Compounds
Related Practice
Textbook Question
1
views
Textbook Question
The iodine monobromide molecule, IBr, has a bond lengthof 249 pm and a dipole moment of 1.21 D. (a) Which atom ofthe molecule is expected to have a negative charge?
Textbook Question
In the following pairs of binary compounds, determine which one is a molecular substance and which one is an ionic substance. Use the appropriate naming convention (for ionic or molecular substances) to assign a name to each compound: (a) SiF4 and LaF3 (for ionic or molecular substances) to assign a name to each compound: (b) FeCl2 and ReCl6 (c) PbCl4 and RbCl.
1
views
Textbook Question
Draw Lewis structures for the following: (a) CH2Cl2 (b) ClCN (c) SF2
Textbook Question
Draw Lewis structures for the following: (d) H2SO4 (H is bonded to O) (e) OF2
Textbook Question
Draw Lewis structures for the following: (f) NH2OH
