(b) If you were to put HBr under very high pressure, so its bond length decreased significantly, would its dipole moment increase, decrease, or stay the same, if you assume that the effective charges on the atoms do not change?

In the following pairs of binary compounds, determine which one is a molecular substance and which one is an ionic substance. Use the appropriate naming convention (for ionic or molecular substances) to assign a name to each compound: (a) TiCl4 and CaF2 (b) ClF3 and VF3
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Ionic vs. Molecular Compounds

Naming Ionic Compounds

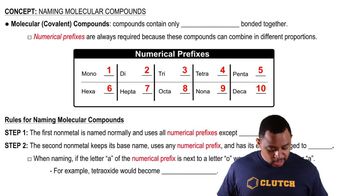
Naming Molecular Compounds
In the following pairs of binary compounds, determine which one is a molecular substance and which one is an ionic substance. Use the appropriate naming convention (for ionic or molecular substances) to assign a name to each compound: (a) SiF4 and LaF3 (for ionic or molecular substances) to assign a name to each compound: (b) FeCl2 and ReCl6 (c) PbCl4 and RbCl.
In the following pairs of binary compounds, determine which one is a molecular substance and which one is an ionic substance. Use the appropriate naming convention (for ionic or molecular substances) to assign a name to each compound: (c) SbCl5 and AlF3.
Draw Lewis structures for the following: (a) CH2Cl2 (b) ClCN (c) SF2
Draw Lewis structures for the following: (d) H2SO4 (H is bonded to O) (e) OF2
