In the following pairs of binary compounds, determine which one is a molecular substance and which one is an ionic substance. Use the appropriate naming convention (for ionic or molecular substances) to assign a name to each compound: (a) TiCl4 and CaF2 (b) ClF3 and VF3

The iodine monobromide molecule, IBr, has a bond lengthof 249 pm and a dipole moment of 1.21 D. (a) Which atom ofthe molecule is expected to have a negative charge?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
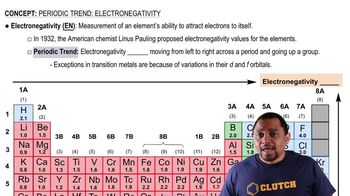
Electronegativity
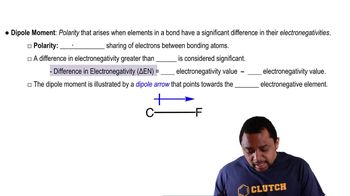
Dipole Moment
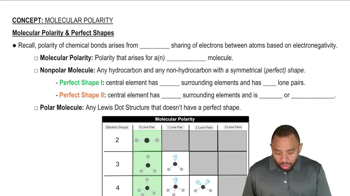
Bond Polarity
Arrange the bonds in each of the following sets in order of increasing polarity: (c) C—S, B—F, N—O.
(a) From the data in Table 8.2, calculate the effective charges on the H atom of the HBr molecule in units of the electronic charge, e.
(b) If you were to put HBr under very high pressure, so its bond length decreased significantly, would its dipole moment increase, decrease, or stay the same, if you assume that the effective charges on the atoms do not change?
In the following pairs of binary compounds, determine which one is a molecular substance and which one is an ionic substance. Use the appropriate naming convention (for ionic or molecular substances) to assign a name to each compound: (a) SiF4 and LaF3 (for ionic or molecular substances) to assign a name to each compound: (b) FeCl2 and ReCl6 (c) PbCl4 and RbCl.
In the following pairs of binary compounds, determine which one is a molecular substance and which one is an ionic substance. Use the appropriate naming convention (for ionic or molecular substances) to assign a name to each compound: (c) SbCl5 and AlF3.
