In the following pairs of binary compounds, determine which one is a molecular substance and which one is an ionic substance. Use the appropriate naming convention (for ionic or molecular substances) to assign a name to each compound: (c) SbCl5 and AlF3.
Ch.8 - Basic Concepts of Chemical Bonding

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 8, Problem 47f
Draw Lewis structures for the following: (f) NH2OH
 Verified step by step guidance
Verified step by step guidance1
Step 1: Determine the total number of valence electrons. Nitrogen (N) has 5 valence electrons, Hydrogen (H) has 1 valence electron, and Oxygen (O) has 6 valence electrons. Since there are 2 Hydrogen atoms and 1 Oxygen atom, the total number of valence electrons is 5 (from N) + 2*1 (from 2 H) + 6 (from O) = 14 valence electrons.
Step 2: Draw a skeleton structure of the molecule. Nitrogen is the least electronegative atom (excluding Hydrogen), so it will be the central atom. Connect the Nitrogen atom to the Oxygen atom and each Hydrogen atom with a single bond. Each single bond represents 2 electrons.
Step 3: Distribute the remaining electrons as lone pairs. Start from the outer atoms. Hydrogen atoms are already filled because they can hold only 2 electrons. So, place the remaining electrons on the Oxygen and Nitrogen atoms. Each atom should have 8 electrons (octet rule) except Hydrogen which can only hold 2 electrons.
Step 4: If there are not enough electrons to give every atom an octet, try multiple bonds (double, triple). In this case, all atoms satisfy the octet rule, so no need for multiple bonds.
Step 5: Verify the structure. Check that the total number of electrons in the Lewis structure is the same as the total number of valence electrons calculated in step 1. Also, make sure each atom satisfies the octet rule (or duet rule for Hydrogen).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Lewis Structures
Lewis structures are diagrams that represent the bonding between atoms in a molecule and the lone pairs of electrons that may exist. They use dots to represent valence electrons and lines to represent bonds between atoms. Understanding how to draw Lewis structures is essential for visualizing molecular geometry and predicting reactivity.
Recommended video:
Guided course

Lewis Dot Structures: Ions
Valence Electrons
Valence electrons are the outermost electrons of an atom and are crucial in determining how atoms bond with each other. The number of valence electrons influences the molecule's structure and stability. For NH2OH, knowing the valence electrons of nitrogen, oxygen, and hydrogen helps in accurately constructing its Lewis structure.
Recommended video:
Guided course
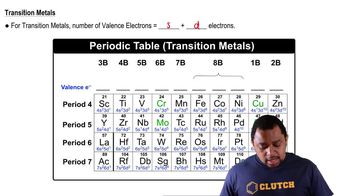
Transition Metals Valence Electrons
Octet Rule
The octet rule states that atoms tend to form bonds in such a way that they each have eight electrons in their valence shell, achieving a stable electron configuration similar to that of noble gases. This rule guides the arrangement of electrons in Lewis structures, ensuring that each atom, particularly carbon, nitrogen, and oxygen, satisfies this rule when possible.
Recommended video:
Guided course
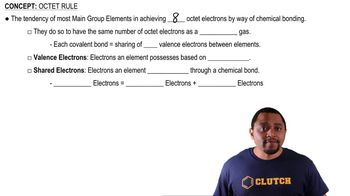
Octet Rule
Related Practice
Textbook Question
1
views
Textbook Question
Draw Lewis structures for the following: (a) CH2Cl2 (b) ClCN (c) SF2
Textbook Question
Draw Lewis structures for the following: (d) H2SO4 (H is bonded to O) (e) OF2
Textbook Question
Write Lewis structures for the following: (a) H2CO (bothH atoms are bonded to C), (b) H2O2, (c) C2F6 (containsa C¬C bond), (d) AsO33 - , (e) H2SO3 (H is bonded to O), (f) NH2Cl.
Textbook Question
Which one of these statements about formal charge is true? (a) Formal charge is the same as oxidation number. (b) To draw the best Lewis structure, you should minimize formal charge. (c) Formal charge takes into account the different electronegativities of the atoms in a molecule. (d) Formal charge is most useful for ionic compounds. (e) Formal charge is used in calculating the dipole moment of a diatomic molecule.
