Some ions do not have a corresponding neutral atom that has the same electron configuration. For each of the following ions, identify the neutral atom that has the same number of electrons and determine if this atom has the same electron configuration. (a) CI−, (b) Sc3+, (c) Fe2+, (d) Zn2+, (e) Sn4+.
Ch.7 - Periodic Properties of the Elements

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 7, Problem 31d
Consider the isoelectronic ions F- and Na+. (d) For isoelectronic ions, how are effective nuclear charge and ionic radius related?
 Verified step by step guidance
Verified step by step guidance1
Understand that isoelectronic ions have the same number of electrons but different numbers of protons.
Recognize that effective nuclear charge (Z_eff) is the net positive charge experienced by an electron in a multi-electron atom, calculated as Z_eff = Z - S, where Z is the atomic number and S is the shielding constant.
For isoelectronic ions, the ion with the higher atomic number (more protons) will have a higher effective nuclear charge because the shielding effect is similar, but the nuclear charge is greater.
A higher effective nuclear charge results in a stronger attraction between the nucleus and the electrons, pulling the electron cloud closer to the nucleus and resulting in a smaller ionic radius.
Apply this understanding to F^- and Na^+: Na^+ has more protons than F^-, so it has a higher effective nuclear charge and a smaller ionic radius.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Isoelectronic Ions
Isoelectronic ions are ions that have the same number of electrons, resulting in identical electron configurations. In this case, F- and Na+ both have 10 electrons, making them isoelectronic. This similarity allows for a direct comparison of their properties, such as ionic radius and effective nuclear charge.
Recommended video:
Guided course

Atoms vs. Ions
Effective Nuclear Charge (Z_eff)
Effective nuclear charge refers to the net positive charge experienced by an electron in a multi-electron atom. It accounts for the shielding effect of inner electrons that reduce the full nuclear charge. For isoelectronic ions, the effective nuclear charge increases with the atomic number, affecting the attraction between the nucleus and the outer electrons.
Recommended video:
Guided course
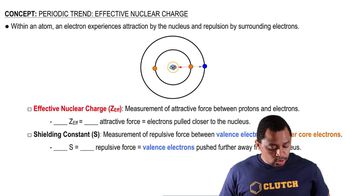
Effective Nuclear Charge
Ionic Radius
Ionic radius is the measure of an ion's size, which can be influenced by its charge and the effective nuclear charge. In isoelectronic ions, the ion with the higher effective nuclear charge will have a smaller ionic radius due to stronger attraction between the nucleus and the electrons. Thus, Na+ will have a smaller radius than F- because Na+ has a higher Z_eff.
Recommended video:
Guided course
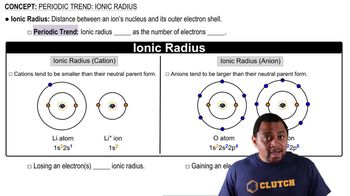
Ionic Radius
Related Practice
Textbook Question
1
views
Textbook Question
Consider the isoelectronic ions F- and Na+. (b) Using Equation 7.1 and assuming that core electrons contribute 1.00 and valence electrons contribute 0.00 to the screening constant, S, calculate Zeff for the 2p electrons in both ions.
Textbook Question
Consider the isoelectronic ions F- and Na+. (c) Repeat this calculation using Slater’s rules to estimate the screening constant, S.
Textbook Question
Consider S, Cl, and K and their most common ions. (a) List the atoms in order of increasing size.
1
views
Textbook Question
Consider S, Cl, and K and their most common ions. (b) List the ions in order of increasing size. (c) Explain any differences in the orders of the atomic and ionic sizes.
